Obicetrapib exhibits favorable physiochemical and pharmacokinetic properties compared to previous cholesteryl ester transfer protein inhibitors: An integrated summary of results from non-human primate studies and clinical trials
- PMID: 39425271
- PMCID: PMC11489133
- DOI: 10.1002/prp2.70010
Obicetrapib exhibits favorable physiochemical and pharmacokinetic properties compared to previous cholesteryl ester transfer protein inhibitors: An integrated summary of results from non-human primate studies and clinical trials
Abstract
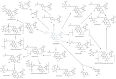
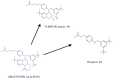
Anacetrapib, a cholesteryl ester transfer protein (CETP) inhibitor previously under development, exhibited an usually extended terminal half-life and large food effect and accumulated in adipose tissue. Other CETP inhibitors have not shown such effects. Obicetrapib, a potent selective CETP inhibitor, is undergoing Phase III clinical development. Dedicated assessments were conducted in pre-clinical and Phase I and II clinical studies of obicetrapib to examine the pharmacokinetic issues observed with anacetrapib. After 9 months of dosing up to 50 mg/kg/day in cynomolgus monkeys, obicetrapib was completely eliminated from systemic circulation and not detected in adipose tissue after a 13-week recovery period. In healthy humans receiving 1-25 mg of obicetrapib, the mean terminal half-life of obicetrapib was 148, 131, and 121 h at 5, 10, and 25 mg, respectively, and food increased plasma levels by ~1.6-fold with a 10 mg dose. At the end of treatment in Phase II trials, mean plasma levels of obicetrapib ranged from 194.5 ng/mL with 2.5 mg to 506.3 ng/mL with 10 mg. Plasma levels of obicetrapib decreased by 92.2% and 98.5% at four and 15 weeks post-treatment, respectively. Obicetrapib shows no clinically relevant accumulation, is minimally affected by food, and has a mean terminal half-life of 131 h for the 10 mg dose. These data support once daily, chronic dosing of obicetrapib in Phase III trials for dyslipidemia management.
Keywords: accumulation; anacetrapib; cholesteryl ester transfer protein; elimination; obicetrapib; pharmacokinetics; toxicokinetic.
© 2024 The Author(s). Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
Figures
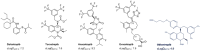
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

