Acylated Anthocyanins From Black Carrots and Their Related Phenolic Acids Diminish Priming and Activation of the NLRP3 Inflammasome in THP-1 Monocytes
- PMID: 39425563
- PMCID: PMC11605781
- DOI: 10.1002/mnfr.202400356
Acylated Anthocyanins From Black Carrots and Their Related Phenolic Acids Diminish Priming and Activation of the NLRP3 Inflammasome in THP-1 Monocytes
Abstract
Scope: Excessive activation of the nucleotide-binding oligomerization domain-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasome contributes to chronic inflammation. Thus, targeting NLRP3 inflammasome activation by anthocyanins may prevent inflammatory diseases. Therefore, the present study determines the influence of a black carrot extract (BCE) with high amounts of acylated anthocyanins and their related phenolic acids on the NLRP3 inflammasome.
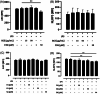
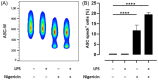
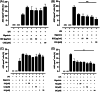
Methods and results: THP-1 monocytes are pretreated with a BCE, cyanidin-3-glucoside (C3G), or hydroxycinnamic acids. NLRP3 inflammasome assembly is initiated by priming THP-1 monocytes with lipopolysaccharide and/or activating the NLRP3 inflammasome with nigericin. Flow cytometry is used to assess apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) speck formation, as well as ASC and NLRP3 protein expression. Caspase-1 activity is measured using a bioluminescent assay, and cytokine concentrations are determined by enzyme-linked immunosorbent assays (ELISA). C3G and phenolic acids diminish ASC and NLRP3 protein expression. In addition, C3G and phenolic acids attenuate ASC speck formation. Furthermore, the BCE and C3G decline caspase-1 activity. Consistently, IL-1β and IL-18 secretion are reduced upon NLRP3 inflammasome activation.
Conclusion: The present study shows that a BCE with high amounts of acylated anthocyanins and their related phenolic acids diminish priming and activation of the NLRP3 inflammasome in THP-1 monocytes.
Keywords: ASC specks; NLRP3 inflammasome; anthocyanin‐rich black carrot extract; phenolic acids.
© 2024 The Author(s). Molecular Nutrition & Food Research published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

