Comparative outcomes of various transplantation platforms, highlighting haploidentical transplants with post-transplantation cyclophosphamide for adult T-cell leukaemia/lymphoma
- PMID: 39425565
- PMCID: PMC11739753
- DOI: 10.1111/bjh.19835
Comparative outcomes of various transplantation platforms, highlighting haploidentical transplants with post-transplantation cyclophosphamide for adult T-cell leukaemia/lymphoma
Abstract
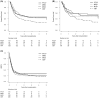
This study retrospectively compared outcomes of various allogeneic haematopoietic cell transplantation (allo-HCT) platforms in patients with adult T-cell leukaemia/lymphoma. Platforms included human leukocyte antigen (HLA)-haploidentical-related donors using post-transplant cyclophosphamide (PTCY), HLA-matched related donors (MRD), HLA-matched unrelated donors (MUD) and cord blood transplantation (CBT). Patients who underwent their first allo-HCT between 2016 and 2021 were included. Outcomes analysed were overall survival (OS), relapse and non-relapse mortality (NRM). Seven hundred patients were included (PTCY, n = 121; MRD, n = 91; MUD, n = 160; CBT, n = 328). With a median follow-up of 794 days for survivors, 2-year OS was 48.1% (PTCY), 48.8% (MRD), 48.4% (MUD) and 34.6% (CBT); the respective 2-year cumulative incidence of relapse was 37.1%, 47.5%, 33.9% and 45.1% and that of NRM was 24.2%, 19.8%, 24.7% and 27.3%. PTCY was associated with delayed platelet engraftment relative to MRD and MUD. There was no increase in the incidence of severe acute or chronic graft-versus-host disease. In the PTCY group, poor performance status was a significant predictor of inferior OS, and infused CD34+ cell numbers of less than 5 × 106/kg were associated with delayed neutrophil and platelet engraftment. These results suggest that allo-HCT with PTCY is a safe and effective platform for patients with adult T-cell leukaemia/lymphoma.
Keywords: adult T‐cell leukaemia/lymphoma; graft‐versus‐host disease; haploidentical haematopoietic stem cell transplantation; post‐transplant cyclophosphamide; prophylaxis.
© 2024 The Author(s). British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
MY: Honoraria from Kyowa Kirin, Daiichi Sankyo, Takeda, Eisai, Chugai, Meiji, Genmab, Bristol‐Myers Squibb, Astellas. KK: Research funding from Chugai, Janssen, Meiji, Novartis, AbbVie, Genmab, Bristol‐Myers Squibb, Gilead Sciences, Eisai, Astellas, Ono, Kyowa Kirin, Nippon Shinyaku, MSD, Takeda, LSI Medience, Japan Blood Products Organization, Honoraria from Chugai, Janssen, Meiji, Novartis, AbbVie, Genmab, Bristol‐Myers Squibb, Gilead Sciences, Eisai, Astellas, Otsuka, Kyowa Kirin, Nippon Shinyaku, MSD, Japan Blood Products Organization. YA: Lecture fee/honorarium from Otsuka Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Novartis Pharma KK, Meiji Seika Pharma Co, Ltd., Janssen Pharmaceutical K.K., Consultant fee from JCR Pharmaceuticals Co., Ltd. and Kyowa Kirin Co., Ltd.
Figures



References
-
- Ishitsuka K, Tamura K. Human T‐cell leukaemia virus type I and adult T‐cell leukaemia‐lymphoma. Lancet Oncol. 2014;15(11):e517–e526. - PubMed
-
- Utsunomiya A, Miyazaki Y, Takatsuka Y, Hanada S, Uozumi K, Yashiki S, et al. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27(1):15–20. - PubMed
-
- Hishizawa M, Kanda J, Utsunomiya A, Taniguchi S, Eto T, Moriuchi Y, et al. Transplantation of allogeneic hematopoietic stem cells for adult T‐cell leukemia: a nationwide retrospective study. Blood. 2010;116(8):1369–1376. - PubMed
-
- Fuji S, Fujiwara H, Nakano N, Wake A, Inoue Y, Fukuda T, et al. Early application of related SCT might improve clinical outcome in adult T‐cell leukemia/lymphoma. Bone Marrow Transplant. 2016;51(2):205–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

