Rational Search for Betaine/GABA Transporter 1 Inhibitors─ In Vitro Evaluation of Selected Hit Compound
- PMID: 39425769
- PMCID: PMC11587516
- DOI: 10.1021/acschemneuro.4c00425
Rational Search for Betaine/GABA Transporter 1 Inhibitors─ In Vitro Evaluation of Selected Hit Compound
Abstract
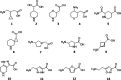
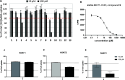
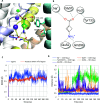
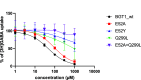
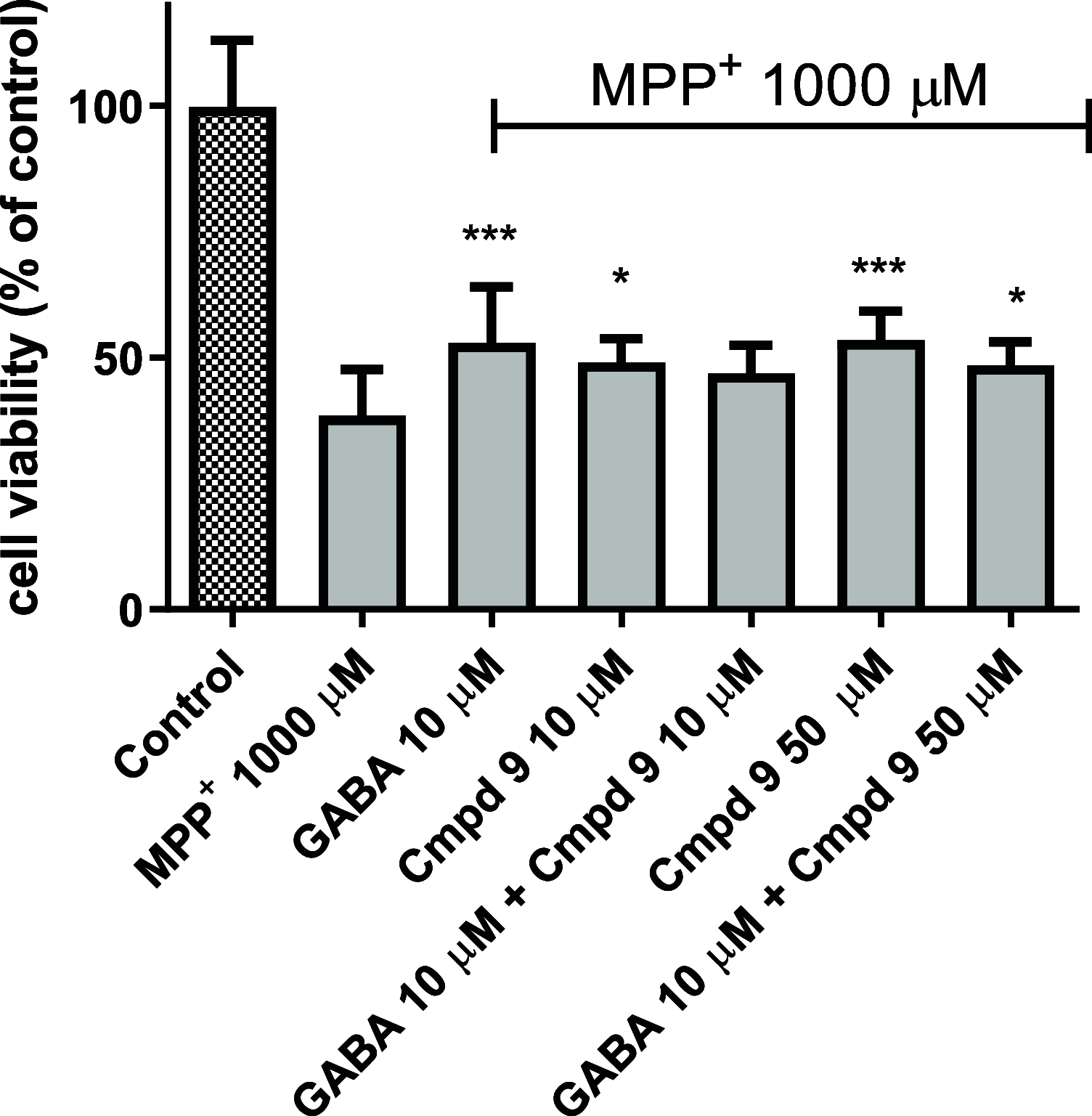
Inhibitory neurotransmission mediated by γ-aminobutyric acid (GABA) plays an important role in maintaining body homeostasis. Disturbances in GABA signaling are implicated in a multitude of neurologic and psychiatric conditions, including epilepsy, ischemia, anxiety, depression, insomnia, and mood disorders. Clinically relevant increases in GABA neurotransmitter level can be achieved by inhibition of its uptake into presynaptic neurons and surrounding glial cells, driven by GABA transporters (GAT1, BGT1, GAT2, and GAT3). Herein, we focused on the search for inhibitors of the BGT1 transporter which is understudied and for which the therapeutic potential of its inhibition is partly unknown. We applied multilevel virtual screening to identify compounds with inhibitory properties. Among selected hits, compound 9 was shown to be a preferential inhibitor of BGT1 (IC50 13.9 μM). The compound also revealed some inhibitory activity against GAT3 (4x lower) while showing no or low activity (IC50 > 100 μM) toward GAT1 and GAT2, respectively. The predicted binding mode of compound 9 was confirmed by mutagenesis studies on E52A, E52Y, Q299L, and E52A+Q299L human BGT1 mutants. Subsequent evaluation showed that the selected hit displayed no affinity toward major GABAA receptor subtypes. Moreover, it was nontoxic when tested on normal human astrocytes and even showed some neuroprotective activity in SH-SY5Y cells. Compound 9 is considered a promising candidate for further evaluation of the therapeutic potential of BGT1 transporter inhibition and the development of novel inhibitors.
Keywords: betaine/GABA transporter 1; biological evaluation; inhibitor; rigid GABA analogue; virtual screening.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Ochoa-de la Paz L. D.; Gulias-Cañizo R.; D′Abril Ruíz-Leyja E.; Sánchez-Castillo H.; Parodí J. The role of GABA neurotransmitter in the human central nervous system, physiology, and pathophysiology. Rev. Mex. Neuroci. 2021, 22, 67–76. 10.24875/RMN.20000050. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

