Immune checkpoint inhibitors in advanced gastroesophageal adenocarcinoma: a series of patient-level meta-analyses in different programmed death-ligand 1 subgroups
- PMID: 39426081
- PMCID: PMC11533044
- DOI: 10.1016/j.esmoop.2024.103962
Immune checkpoint inhibitors in advanced gastroesophageal adenocarcinoma: a series of patient-level meta-analyses in different programmed death-ligand 1 subgroups
Abstract
Background: While the benefit of immune checkpoint inhibitors (ICI) is well established in programmed death-ligand 1 high (PD-L1high) advanced gastroesophageal adenocarcinoma (GEAC), there remains significant controversy about their benefit in PD-L1low GEAC. To elucidate the benefit of ICI in PD-L1low and PD-L1negative GEAC, we conducted an analysis leveraging individual patient data (IPD) extracted from Kaplan-Meier (KM) plots of pivotal trials.
Methods: KM curves from randomized clinical trials investigating the efficacy of ICI for advanced GEAC were extracted from published articles. IPD were extracted from the reported curves, and, in the case of unreported KM plots, KMSubtraction was used to retrieve survival data. A patient-level meta-analysis was conducted for PD-L1low tumors.
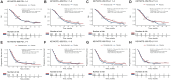
Results: In the human epidermal growth factor receptor 2 (HER2)-negative setting, pooled PD-L1 combined positive score (CPS) 1-4 subgroup KM plots from KEYNOTE-859, CHECKMATE-649, and RATIONALE-305 showed a modest overall survival (OS) benefit with the addition of an anti-programmed cell death protein 1 (anti-PD-1) agent [hazard ratio (HR) 0.868, P = 0.018]. Similarly, a modest OS benefit was shown by our IPD meta-analysis of PD-L1 CPS 1-9 subgroups from KEYNOTE-859, KEYNOTE-062, and RATIONALE-305 (HR 0.840, P = 0.002.) Conversely, when CPS 5-9 subgroup KM plots from KEYNOTE-859 and RATIONALE-305 were pooled together, no significant OS benefit was found in the ICI-chemotherapy arm (HR 0.867, P = 0.181), although this subgroup was relatively small.
Conclusions: In PD-L1low HER-2 negative GEAC, the benefit of first-line ICI is modest, yet significant. Further translational work is warranted to better select patients who could benefit from immunotherapy in this setting. Meanwhile, alternative therapeutic options such as zolbetuximab in Claudin18.2-positive disease must be taken into account.
Keywords: PD-L1; gastroesophageal adenocarcinoma; immune checkpoint inhibitors.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- FDA FDA approves nivolumab in combination with chemotherapy for metastatic gastric cancer and esophageal adenocarcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-appro... Available at.
-
- BMS Bristol Myers Squibb receives European Commission approval for Opdivo (nivolumab) + chemotherapy for patients with HER2 negative, advanced or metastatic gastric, gastroesophageal junction or esophageal adenocarcinoma whose tumors express PD-L1 with CPS ≥5. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Receives-Eur... Available at.
-
- Rha S.Y., Oh D.-Y., Yañez P., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(11):1181–1195. - PubMed
-
- FDA FDA approves pembrolizumab for esophageal or GEJ carcinoma. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-appro... Available at.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

