NoxO1 regulates EGFR signaling by its interaction with Erbin
- PMID: 39426288
- PMCID: PMC11536020
- DOI: 10.1016/j.redox.2024.103396
NoxO1 regulates EGFR signaling by its interaction with Erbin
Abstract
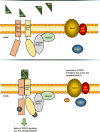
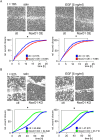
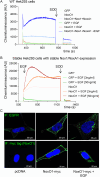
NADPH oxidase organizer 1 (NoxO1) is a scaffold cytoplasmic subunit of the reactive oxygen species (ROS) forming Nox1 complex and involved in angiogenesis, differentiation, and atherosclerosis. We found that overexpression of NoxO1 without simultaneous overexpression of any other component of the active Nox1 complex inhibited EGF-induced wound closure and signaling, while NoxO1 KO yielded the opposite effect. Accordingly, we hypothesize NoxO1 to exert Nox1 independent functions. Using the BioID technique, we identified ErbB2 interacting protein (Erbin) as novel interaction partner of NoxO1. Colocalization of NoxO1 with EGFR, as well as with Erbin validated this finding. EGF treatment interrupted colocalization of NoxO1 and EGFR. EGF mediated kinase activation was delayed in NoxO1 overexpressing cells, while knockout of NoxO1 had the opposite effect. In conclusion, Erbin was identified as a novel NoxO1 interacting protein. Through the subsequent interaction of NoxO1 and EGFR, NoxO1 interferes with EGF signaling. The results of this study suggest a potential role of NoxO1 as an adaptor protein with functions beyond the well-established enabling of Nox1 mediated ROS formation.
Keywords: EGF signaling; Erbin; NADPH oxidase; NoxO1.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

