Iron-loaded cancer-associated fibroblasts induce immunosuppression in prostate cancer
- PMID: 39426954
- PMCID: PMC11490570
- DOI: 10.1038/s41467-024-53233-1
Iron-loaded cancer-associated fibroblasts induce immunosuppression in prostate cancer
Abstract
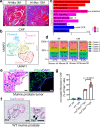
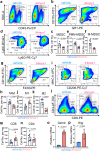
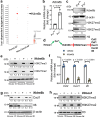
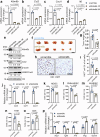
Iron is an essential biomineral in the human body. Here, we describe a subset of iron-loaded cancer-associated fibroblasts, termed as FerroCAFs, that utilize iron to induce immunosuppression in prostate cancer and predict an unfavorable clinical outcome. FerroCAFs secrete myeloid cell-associated proteins, including CCL2, CSF1 and CXCL1, to recruit immunosuppressive myeloid cells. We report the presence of FerroCAFs in prostate cancer from both mice and human, as well as in human lung and ovarian cancers, and identify a conserved cell surface marker, the poliovirus receptor. Mechanistically, the accumulated iron in FerroCAFs is caused by Hmox1-mediated iron release from heme degradation. The intracellular iron activates the Kdm6b, an iron-dependent epigenetic enzyme, to induce an accessible chromatin state and transcription of myeloid cell-associated protein genes. Targeting the FerroCAFs by inhibiting the Hmox1/iron/Kdm6b signaling axis incurs anti-tumor immunity and tumor suppression. Collectively, we report an iron-loaded FerroCAF cluster that drives immunosuppression through an iron-dependent epigenetic reprogramming mechanism and reveal promising therapeutic targets to boost anti-tumor immunity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Abbate, V. & Hider, R. Iron in biology. Metallomics Integr. Biometal Sci.9, 1467–1469 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NSFC U23A20454/National Natural Science Foundation of China (National Science Foundation of China)
- NSFC 82372873/National Natural Science Foundation of China (National Science Foundation of China)
- NSFC 81972404/National Natural Science Foundation of China (National Science Foundation of China)
- NSFC 82472909/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

