Enhanced docetaxel therapeutic effect using dual targeted SRL-2 and TA1 aptamer conjugated micelles in inhibition Balb/c mice breast cancer model
- PMID: 39427007
- PMCID: PMC11490543
- DOI: 10.1038/s41598-024-75042-8
Enhanced docetaxel therapeutic effect using dual targeted SRL-2 and TA1 aptamer conjugated micelles in inhibition Balb/c mice breast cancer model
Abstract
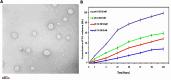
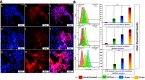
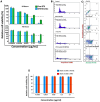
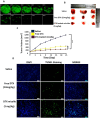
Effective targeting and delivery of large amounts of medications into the cancer cells enhance their therapeutic efficacy through saturation of cellular defensive mechanisms, which is the most privilege of nano drug delivery systems (NDDS) compared to traditional approaches. Herein, we designed dual-pH/redox responsive DTX-loaded poly (β-amino ester) (PBAS) micelles decorated with a chimeric peptide and TA1 aptamer. In vitro and in vivo results demonstrated that the designed nanoplatform possessed an undetectable nature in the blood circulation, but after exposure to the tumor microenvironment (TME) of 4T1 breast cancer, it suddenly changed into dual targeting nanoparticles (NPs) (containing two ligands, SRL-2 and TA1 aptamer). The dual targeting NPs destruction in the high GSH and low pH conditions of the cancer cells led to amplified DTX release (around 70% at 24 h). The IC50 value of DTX-loaded MMP-9 sensitive heptapeptide/TA1 aptamer-modified poly (β-amino ester) (MST@PBAS) micelles and free DTX after 48 h of exposure was determined to be 1.5 µg/ml and 7.5 µg/ml, respectively. The nano-formulated DTX exhibited cytotoxicity that was 5-fold stronger than free DTX (Pvalue˂0.001). Cell cycle assay test results showed that following exposure to MST@PBAS micelles, a considerable rise in the sub G1 population (48%) suggested that apoptosis by cell cycle arrest had occurred. DTX-loaded MST@PBAS micelles revealed significantly higher (Pvalue ˂ 0.001) levels of early apoptosis (59.8%) than free DTX (44.7%). Interestingly, in vitro uptake studies showed a significantly higher TME accumulation of dual targeted NPs (6-fold) compared to single targeted NPs (Pvalue < 0.001) which further confirmed by in vivo biodistribution and fluorescent TUNEL assay experiments. NPs treated groups demonstrated notable tumor growth inhibition in 4T1 tumor bearing Balb/c mice by only 1/10th of the DTX therapeutic dose (TD) as a drug model. In conclusion, cleverly designed nanostructures here demonstrated improved anticancer effects by enhancing tumor targeting, delivering chemotherapeutic agents more accurately, promoting drug release, reducing the therapeutic dosage, and lowering side effects of anticancer drugs.
Keywords: DTX; Delivery; Drug resistance; GSH/pH responsive; SRL-2; Smart; TA1 aptamer.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
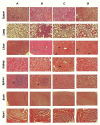
Figures

References
-
- El-Tanani, M. et al. Unraveling the tumor microenvironment: Insights into cancer metastasis and therapeutic strategies. Cancer Lett., 216894. (2024). - PubMed
-
- Corbeau, A. et al. Correlations between bone marrow radiation dose and hematologic toxicity in locally advanced cervical cancer patients receiving chemoradiation with cisplatin: A systematic review. Radiother. Oncol.164, 128–137 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

