Can switching from cigarettes to heated tobacco products reduce consequences of pulmonary infection?
- PMID: 39427167
- PMCID: PMC11491011
- DOI: 10.1186/s12931-024-02992-y
Can switching from cigarettes to heated tobacco products reduce consequences of pulmonary infection?
Abstract
Rationale: While tobacco industry data suggests that switching from combustible cigarettes to heated tobacco products (HTPs), like IQOS, may reduce the users' exposure to respiratory toxicants, it is not known if using HTPs impacts the outcomes of acute respiratory infections.
Objectives: Does switching from cigarettes to HTPs improve responses to pulmonary infection.
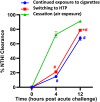
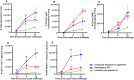
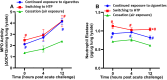
Methods: We conducted experiments in which 3 groups of mice were pre-exposed to cigarette smoke for 8 weeks, followed by 8-week exposure to (1) HTPs (tobacco product switching), (2) air (smoking cessation), or (3) continued exposure to cigarette smoke. Pulmonary bacterial clearance and surrogate markers of lung damage were assessed as study outcomes.
Main results: Significantly compromised clearance of bacteria from the lungs post-acute challenge occurred in both the switching group and in mice continuously exposed to cigarette smoke. Bacterial clearance, inflammatory T-cell infiltration into the lungs, and albumin leak improved at 12 h post-acute challenge in the switching group compared to mice continuously exposed to cigarette smoke. Bacterial clearance, total lung immune-cell infiltration, inflammatory T-cell infiltration into the lungs, the content of total proteins in the BAL, and albumin leak measured post-acute challenge were compromised in the switching group compared to mice in the cessation group. Switching from cigarettes to HTPs did not improve lung myeloperoxidase and neutrophil elastase levels (markers for lung inflammation and damage), which, however, were significantly reduced in the cessation group.
Conclusions: This study reveals only a modest improvement in respiratory infection outcomes after switching exposure from cigarettes to HTPs and significantly compromised outcomes compared to a complete cessation of exposure to all tobacco products.
Keywords: Combustible cigarettes; Heated tobacco products (HTPs); IQOS; NTHI; Respiratory infection; Smoking cessation; Tobacco product switching.
© 2024. The Author(s).
Conflict of interest statement
MLG received a research grant from Pfizer and served as a member of the Scientific Advisory Board to Johnson & Johnson outside of this work; he has also consulted with the US Food and Drug Administration, World Health Organization, and Campaign for Tobacco-Free Kids on the toxicity of tobacco products and tobacco control policies; MLG is also a Member of the IASLC Tobacco Control and Smoking Cessation Committee; and AACR Tobacco Product and Cancer Subcommittee. Others report no conflict of interest.
Figures



References
-
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US). 2010. https://www.ncbi.nlm.nih.gov/books/NBK53017/ - PubMed
-
- U.S. Department of Health and Human Services. The Health consequences of Smoking—50 years of progress: a report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [accessed 2016 Dec 20].
-
- Vellappally S, Fiala Z, Smejkalová J, Jacob V, Somanathan R. Smoking related systemic and oral diseases. Acta Med (Hradec Kralove). 2007;50(3):161–6. - PubMed
-
- Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9(5):377–84. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

