A genetic and microscopy toolkit for manipulating and monitoring regeneration in Macrostomum lignano
- PMID: 39427313
- PMCID: PMC12435804
- DOI: 10.1016/j.celrep.2024.114892
A genetic and microscopy toolkit for manipulating and monitoring regeneration in Macrostomum lignano
Abstract
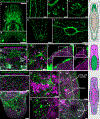
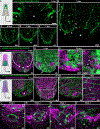
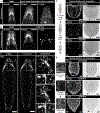
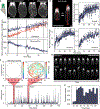
Live imaging of regenerative processes can reveal how animals restore their bodies after injury through a cascade of dynamic cellular events. Here, we present a comprehensive toolkit for live imaging of tissue regeneration in the flatworm Macrostomum lignano, including a high-throughput cloning pipeline, targeted cellular ablation, and advanced microscopy solutions. Using tissue-specific reporter expression, we examine how various structures regenerate. Enabled by a custom luminescence/fluorescence microscope, we overcome intense stress-induced autofluorescence to demonstrate genetic cellular ablation and reveal the limited regenerative capacity of neurons and their essential role during wound healing, contrasting muscle cells' rapid regeneration after ablation. Finally, we build an open-source tracking microscope to continuously image freely moving animals throughout the week-long process of regeneration, quantifying kinetics of wound healing, nerve cord repair, body regeneration, growth, and behavioral recovery. Our findings suggest that nerve cord reconnection is highly robust and proceeds independently of regeneration.
Keywords: CP: Stem cell research; flatworms; live imaging; luminescence; nervous system; regeneration; transgenics.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests H.L. and M.P. are co-founders of Cephla, commercializing the Squid platform.
Figures


References
-
- Tanaka EM (2016). The molecular and cellular choreography of appendage regeneration. Cell 165, 1598–1608. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

