Enhancing antibacterial efficacy through macrocyclic host complexation of fluoroquinolone antibiotics for overcoming resistance
- PMID: 39428392
- PMCID: PMC11491488
- DOI: 10.1038/s41598-024-73568-5
Enhancing antibacterial efficacy through macrocyclic host complexation of fluoroquinolone antibiotics for overcoming resistance
Abstract
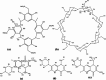
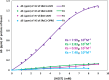
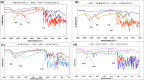
The use of supramolecular assemblies in pharmaceuticals has garnered significant interest. Recent studies have shown that the activities of antibacterial agents can be enhanced through complexation with cyclic oligomers and metal ions. Notably, these complexes sometimes possess greater therapeutic properties than the parent drugs. To develop microbiologically potent supramolecular drugs, the complexation of macrocyclic hosts with fluoroquinolone (FQ) antibiotics was investigated. FQs are a successful family of antibiotics that target the bacterial enzymes DNA gyrase and DNA topoisomerase IV, leading to bacterial cell death through the inhibition of DNA synthesis. However, antibiotic resistance resulting from the repeated use of FQs over time has limited their effectiveness against resistant pathogens. To overcome this issue, the encapsulation of FQs in polyphenolic macrocycles was investigated. This study highlights resorcinarene, a polyphenolic host with antibacterial properties, and its ability to chemically interact with FQs. The inclusion complexation process was analyzed using NMR and FTIR techniques. The binding constants determined by 1H-NMR titration revealed that levofloxacin forms more stable complexes with resorcinarene than with β-cyclodextrin, which aligned with MD simulations. Assessment of the geometric characteristics of the inclusion complexes using 2D NMR analysis confirmed that different moieties of various FQs can fit into a single host cavity and improve activity against gram-negative bacteria. Overall, these findings suggest that encapsulation in polyphenolic macrocycles is a promising strategy for utilizing FQs against antibiotic-resistant bacteria.
Keywords: Antibacterial efficacy; Antibiotic resistance; Antibiotics; Fluroquinolones; Macrocyclic host–guest complex; Resistant bacteria; Supramolecular interactions.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
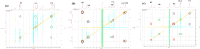
References
-
- Levy, S. B. & Bonnie, M. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med.10, S122–S129. 10.1038/nm1145 (2004). - PubMed
-
- L, C. A. & Adhar, M. Compositions and methods for affecting virulence determinants in bacteria (2008).
-
- Nathan, C. & Cars, O. Antibiotic resistance—problems, progress, and prospects. N. Engl. J. Med.371, 1761–1763. 10.1056/NEJMp1408040 (2014). - PubMed
-
- Walsh, C. Where will new antibiotics come from? Nat. Rev. Microbiol.1, 65–70. 10.1038/nrmicro727 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

