Alleviating rheumatoid arthritis with a photo-pharmacotherapeutic glycan-integrated nanogel complex for advanced percutaneous delivery
- PMID: 39428483
- PMCID: PMC11492540
- DOI: 10.1186/s12951-024-02877-8
Alleviating rheumatoid arthritis with a photo-pharmacotherapeutic glycan-integrated nanogel complex for advanced percutaneous delivery
Abstract
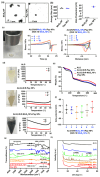
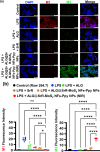
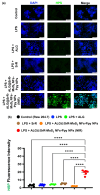
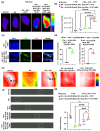
The prospective of percutaneous drug delivery (PDD) mechanisms to address the limitations of oral and injectable treatment for rheumatoid arthritis (RA) is increasing. These limitations encompass inadequate compliance among patients and acute gastrointestinal side effects. However, the skin's intrinsic layer can frequently hinder the percutaneous dispersion of RA medications, thus mitigating the efficiency of drug delivery. To circumvent this constraint, we developed a strontium ranelate (SrR)-loaded alginate (ALG) phototherapeutic hydrogel to assess its effectiveness in combating RA. Our studies revealed that this SrR-loaded ALG hydrogel incorporating photoelectrically responsive molybdenum disulfide nanoflowers (MoS2 NFs) and photothermally responsive polypyrrole nanoparticles (Ppy NPs) to form ALG@SrR-MoS2 NFs-Ppy NPs demonstrated substantial mechanical strength, potentially enabling delivery of hydrophilic therapeutic agents into the skin and significantly impeding the progression of RA. Comprehensive biochemical, histological, behavioral, and radiographic analyses in an animal model of zymosan-induced RA demonstrated that the application of these phototherapeutic ALG@SrR-MoS2 NFs-Ppy NPs effectively reduced inflammation, increased the presence of heat shock proteins, regulatory cluster of differentiation M2 macrophages, and alleviated joint degeneration associated with RA. As demonstrated by our findings, treating RA and possibly other autoimmune disorders with this phototherapeutic hydrogel system offers a distinctive, highly compliant, and therapeutically efficient method.
Keywords: Immunomodulation; Molybdenum disulfide; Phototherapy; Polypyrrole; Rheumatoid arthritis.
© 2024. The Author(s).
Conflict of interest statement
The authors affirmed that they have no competing interests to declare.
Figures






References
-
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–38. 10.1016/S0140-6736(16)30173-8. - PubMed
-
- Combe B, Landewe R, Daien CI, Hua C, Aletaha D, Álvaro-Gracia JM, Bakkers M, Brodin N, Burmester GR, Codreanu C, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis. 2017;76:948–59. 10.1136/annrheumdis-2016-210602. - PubMed
-
- Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320:1360–72. 10.1001/jama.2018.13103. - PubMed
-
- Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–16. 10.1016/j.ejmech.2018.09.027. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

