MOG-specific CAR Tregs: a novel approach to treat multiple sclerosis
- PMID: 39428507
- PMCID: PMC11490997
- DOI: 10.1186/s12974-024-03262-w
MOG-specific CAR Tregs: a novel approach to treat multiple sclerosis
Abstract
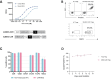
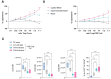
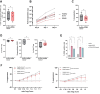
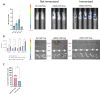
Multiple sclerosis (MS) is an autoimmune disease affecting the central nervous system (CNS) with the immune system attacking myelin sheaths leading to neuronal death. While several disease-modifying therapies are available to treat MS, these therapies are not universally effective and do not stop disease progression. More personalized long-term treatment options that target specific aspects of the disease, such as reducing relapse frequency, delaying disability accumulation, and addressing symptoms that impact daily functioning, as well as therapies that can promote neuroprotection and repair are needed. Chimeric Antigen Receptor (CAR) Tcell therapies have revolutionized cancer treatment by intravenously (IV) administering a defined dose of T cells with high specificity provided by the CAR. An autologous CAR T cell therapy using suppressive regulatory T cells (Tregs) inducing long-lasting tolerance would be the ideal treatment for patients. Hence, we expanded the application of CAR-T cells by introducing a CAR into Tregs to treat MS patients. We developed a myelin oligodendrocyte glycoprotein (MOG)-specific CAR Treg cell therapy for patients with MS. MOG is expressed on the outer membrane of the myelin sheath, the insulating layer the forms around nerves, making it an ideal target for CAR Treg therapy. Our lead candidate is a 2nd generation CAR, composed of an anti-MOG scFv screened from a large human library. In vitro, we demonstrated CAR-dependent functionality and showed efficacy in vivo using a passive EAE mouse model. Additionally, the MOG-CAR Tregs have very low tonic signaling with a desirable signal-to-noise ratio resulting in a highly potent CAR. In summary our data suggest that MOG-CAR Tregs are a promising MS treatment option with the potential to induce long-lasting tolerance in patients.
Keywords: CAR-Treg; Cell therapy; MOG; Multiple sclerosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Noseworthy John H. Progress in determining the causes and treatment of multiple sclerosis. Nature. 1999;399(6738Suppl):A40–47. - PubMed
-
- Raine CS, Cross AH. Axonal dystrophy as a consequence of long-term demyelination. Lab Invest. 1989;60(5):714–25. - PubMed
-
- Martin R, et al. Molecular Mimicry and Antigen-specific T cell responses in multiple sclerosis and chronic CNS Lyme Disease. J Autoimmun. 2001;16(3):187–92. - PubMed
-
- O’Connor KC, Bar-or A, Hafler DA. The neuroimmunology of multiple sclerosis: possible roles of T and B lymphocytes in immunopathogenesis. J Clin Immunol. 2001;21(2):81–92. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

