Viscous shear is a key force in Drosophila ventral furrow morphogenesis
- PMID: 39428993
- PMCID: PMC11586522
- DOI: 10.1242/dev.202892
Viscous shear is a key force in Drosophila ventral furrow morphogenesis
Abstract
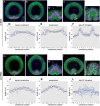
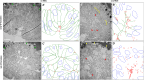
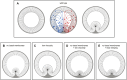
Ventral furrow (VF) formation in Drosophila melanogaster is an important model of epithelial folding. Previous models of VF formation require cell volume conservation to convert apically localized constriction forces into lateral cell elongation and tissue folding. Here, we have investigated embryonic morphogenesis in anillin knockdown (scra RNAi) embryos, where basal cell membranes fail to form and therefore cells can lose cytoplasmic volume through their basal side. Surprisingly, the mesoderm elongation and subsequent folding that comprise VF formation occurred essentially normally. We hypothesized that the effects of viscous shear may be sufficient to drive membrane elongation, providing effective volume conservation, and thus driving tissue folding. Since this hypothesis may not be possible to test experimentally, we turned to a computational approach. To test whether viscous shear is a dominant force for morphogenesis in vivo, we developed a 3D computational model incorporating both accurate cell and tissue geometry, and experimentally measured material parameters. Results from this model demonstrate that viscous shear generates sufficient force to drive cell elongation and tissue folding in vivo.
Keywords: Drosophila; Anillin; Gastrulation; Morphogenesis; Shear; Viscosity.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Campos-Ortega, J. A. and Hartenstein, V. (1985). The Embryonic Development of Drosophila melanogaster. Berlin: Springer.
-
- Cheikh, M. I., Tchoufag, J., Osterfield, M., Dean, K., Bhaduri, S., Zhang, C., Mandadapu, K. K. and Doubrovinski, K. (2023). A comprehensive model of Drosophila epithelium reveals the role of embryo geometry and cell topology in mechanical responses. eLife 12, e85569. 10.7554/eLife.85569.sa2 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

