Bacterial extracellular vesicles as intranasal postbiotics: Detailed characterization and interaction with airway cells
- PMID: 39429019
- PMCID: PMC11491762
- DOI: 10.1002/jev2.70004
Bacterial extracellular vesicles as intranasal postbiotics: Detailed characterization and interaction with airway cells
Abstract
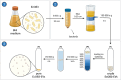
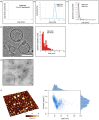
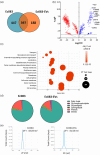
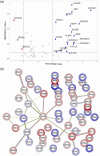
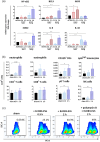
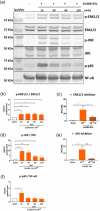
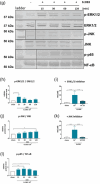
Escherichia coli A0 34/86 (EcO83) is a probiotic strain used in newborns to prevent nosocomial infections and diarrhoea. This bacterium stimulates both pro- and anti-inflammatory cytokine production and its intranasal administration reduces allergic airway inflammation in mice. Despite its benefits, there are concerns about the use of live probiotic bacteria due to potential systemic infections and gene transfer. Extracellular vesicles (EVs) derived from EcO83 (EcO83-EVs) might offer a safer alternative to live bacteria. This study characterizes EcO83-EVs and investigates their interaction with host cells, highlighting their potential as postbiotic therapeutics. EcO83-EVs were isolated, purified, and characterised following the Minimal Information of Studies of Extracellular Vesicles (MISEV) guidelines. Ex vivo studies conducted in human nasal epithelial cells showed that EcO83-EVs increased the expression of proteins linked to oxidative stress and inflammation, indicating an effective interaction between EVs and the host cells. Further in vivo studies in mice demonstrated that EcO83-EVs interact with nasal-associated lymphoid tissue, are internalised by airway macrophages, and stimulate neutrophil recruitment in the lung. Mechanistically, EcO83-EVs activate the NF-κΒ signalling pathway, resulting in the nitric oxide production. EcO83-EVs demonstrate significant potential as a postbiotic alternative to live bacteria, offering a safer option for therapeutic applications. Further research is required to explore their clinical use, particularly in mucosal vaccination and targeted immunotherapy strategies.
Keywords: EVs; Ec083; NF‐κΒ signalling; bacterial extracellular vesicles; macrophage; nitric oxide; postbiotics; probiotic.
© 2024 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors report no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
- PPN/BAT/2021/1/00004/U/00001/Narodowa Agencja Wymiany Akademickiej
- CZ.02.01.01/00/22_010/0008115/OP JAK project MSCA fellowships CZ-UK2
- 23-04050L/Grantová Agentura České Republiky
- Danube-Allergy Cluster (P17)/Amt der NÖ Landesregierung
- CZ 04/2024/OeAD-GmbH
- CZ 07/2023/OeAD-GmbH
- CZ 15/2023/OeAD-GmbH
- RS 08/2022/OeAD-GmbH
- 10.47379/LS20025/Vienna Science and Technology Fund
- LM/29/SM/SciMat and qLife Priority Research Area under Strategic Programme Excellence Initiative "Laboratories for the Young"
- P 34867/Austrian Science Fund
- 101066450/HORIZON EUROPE Marie Sklodowska-Curie Actions
- CZ.02.01.01/00/22_008/0004597/Ministry of Education, Youth and Sports of the Czech Republic
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

