Catastrophic Antiphospholipid Syndrome: A Review of Current Evidence and Future Management Practices
- PMID: 39429267
- PMCID: PMC11490264
- DOI: 10.7759/cureus.69730
Catastrophic Antiphospholipid Syndrome: A Review of Current Evidence and Future Management Practices
Abstract
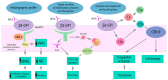
Antiphospholipid syndrome (APS) is an autoimmune disorder characterized by blood clots and pregnancy complications due to antiphospholipid antibodies. Catastrophic APS (CAPS), a severe variant, leads to multiorgan failure and is often fatal. Pathogenesis involves antiphospholipid antibodies, particularly anti-beta-2-glycoprotein I (aβ2GPI), which trigger endothelial cell (EC) activation, cytokine release, and a prothrombotic state. Infections, surgeries, and other triggers can precipitate CAPS, leading to widespread microthromboses and systemic inflammatory responses. CAPS predominantly affects younger patients and those with systemic lupus erythematosus (SLE), with a high mortality rate, though recent treatment advances have improved survival. Diagnosing CAPS involves identifying clinical manifestations, including rapid organ involvement and small vessel occlusions, confirmed by histopathology and high antiphospholipid antibody levels. The CAPS registry data indicate that commonly affected organs include kidneys, lungs, central nervous system, and the heart, with a high prevalence of lupus anticoagulant and anticardiolipin antibodies (aCL). Current management strategies focus on therapeutic anticoagulation, immunosuppressive therapies like corticosteroids, and adjunct treatments such as plasmapheresis and intravenous immunoglobulin (IVIG). Early use of glucocorticoids and combination therapy has significantly improved outcomes. In life-threatening cases, especially with microangiopathy, experts recommend performing plasma exchange (PE). Patients with associated autoimmune conditions or refractory cases may receive cyclophosphamide (CY) and rituximab while considering PE for treatment. Maintenance of anticoagulation with an appropriate international normalized ratio (INR) is crucial to prevent recurrence. This article reviews the pathogenesis and epidemiology of CAPS. It also examines the current management strategies, and discusses the challenges and controversies associated with these strategies. It hereafter offers recommendations for future management and outlines directions for further research.
Keywords: anti coagulation; antiphospholipid antibody syndrome; blood clots; catastrophic antiphospholipid syndrome; multiorgan system failure; thrombosis.
Copyright © 2024, Okunlola et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Update on the diagnosis, treatment, and prognosis of the catastrophic antiphospholipid syndrome. Cervera R. Curr Rheumatol Rep. 2010;12:70–76. - PubMed
-
- 14th International Congress on Antiphospholipid Antibodies Task Force report on catastrophic antiphospholipid syndrome. Cervera R, Rodríguez-Pintó I, Colafrancesco S, et al. Autoimmun Rev. 2014;13:699–707. - PubMed
-
- International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) Miyakis S, Lockshin MD, Atsumi T, et al. J Thromb Haemost. 2006;4:295–306. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
