Leukocyte-Poor Platelet-Rich Plasma for the Management of Knee Osteoarthritis: A Retrospective Study With 12 Months of Follow-Up
- PMID: 39429345
- PMCID: PMC11488677
- DOI: 10.7759/cureus.69662
Leukocyte-Poor Platelet-Rich Plasma for the Management of Knee Osteoarthritis: A Retrospective Study With 12 Months of Follow-Up
Abstract
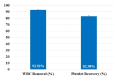
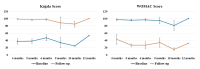
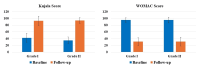
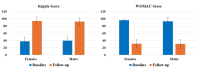
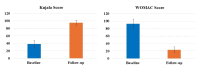
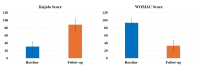
Introduction The knee, the most frequently affected joint in osteoarthritis (OA), impacts the life quality of millions of individuals globally, resulting in a considerable healthcare burden. Conservative treatments are preferred, turning to surgical intervention when necessary. Nonetheless, these conventional modalities have drawbacks. Recently, the use of regenerative medicine therapies, including autologous peripheral blood-derived orthobiologics (APBOs), such as leukocyte-poor platelet-rich plasma (LP-PRP), has evolved and demonstrated the ability to manage knee OA. The primary objective of this investigation was to evaluate the efficacy of LP-PRP via widely used patient-reported outcome measures (PROMs) in grade I or II (on the Kellgren-Lawrence scale) knee OA patients. The secondary objective was to characterize the formulated LP-PRP and determine the efficiency of the leukodepletion filter used for leukocyte removal and platelet recovery. Methods This investigation was a retrospective analysis of data collected from patients treated at a single center over a period of 15 months. Data from 40 patients included in this study were intra-articularly injected with 3mL of formulated LP-PRP under ultrasound guidance. PROMs questionnaires, including Kujala and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores, were used and responses were documented at baseline and up to 12 months follow-up. The characterization of the formulated LP-PRP and the efficiency of the leukodepletion filter in removing leukocytes and recovering platelets were assessed via complete blood count (CBC) analysis. Results The intra-articular administration of LP-PRP resulted in statistically significant improvements in Kujala and WOMAC scores in patients with Grade I or II OA of the knee at all follow-up time points (four to 12 months) compared to the respective baseline scores. The subgroup analysis showed significant improvements in Kujala and WOMAC scores in both male and female grade I or II knee OA patients with or without comorbidities, including diabetes and/or hypertension. The characterization of formulated PRP showed platelet concentration to be at least 6x compared to the baseline whole blood levels, the absolute platelet count to be at least 5 billion, and total leukocytes, lymphocytes, neutrophils, and RBCs were depleted by over 88%, 82%, 98%, and 98%, respectively. In addition, the utilization of the PuriBlood leukocyte reduction filter (Puriblood Medical Co. Ltd., Baoshan Township, Taiwan) led to the depletion of approximately 93% of leukocytes and the recovery of about 83% of platelets. Conclusions Administration of LP-PRP resulted in significant improvements in pain and function of patients suffering from grade I or II OA of the knee. In addition, the leukodepletion filter used to formulate LP-PRP, successfully resulted in the depletion of leukocytes while recovering the platelets. More sufficiently powered, multi-center, prospective, non-randomized, and randomized controlled trials with long-term follow-up are needed to further establish the effectiveness of this formulation in knee OA patients.
Keywords: autologous peripheral blood-derived orthobiologics; knee osteoarthritis; leukocyte-poor platelet rich plasma; lp prp; patient reported outcome measures; platelet-rich plasma; proms; prp; regenerative medicine.
Copyright © 2024, Gupta et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Institutional Ethics Committee, Sri Lakshmi Narayana Institute of Medical Sciences, Puducherry, India issued approval IEC/C-01/2024, dated June 25, 2024. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Osteoarthritis in 2020 and beyond: a Lancet Commission. Hunter DJ, March L, Chew M. Lancet. 2020;396:1711–1712. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
