A method framework of semi-automatic knee bone segmentation and reconstruction from computed tomography (CT) images
- PMID: 39429595
- PMCID: PMC11485355
- DOI: 10.21037/qims-24-821
A method framework of semi-automatic knee bone segmentation and reconstruction from computed tomography (CT) images
Abstract
Background: Accurate delineation of knee bone boundaries is crucial for computer-aided diagnosis (CAD) and effective treatment planning in knee diseases. Current methods often struggle with precise segmentation due to the knee joint's complexity, which includes intricate bone structures and overlapping soft tissues. These challenges are further complicated by variations in patient anatomy and image quality, highlighting the need for improved techniques. This paper presents a novel semi-automatic segmentation method for extracting knee bones from sequential computed tomography (CT) images.
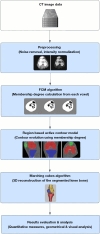
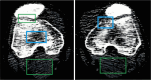
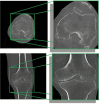
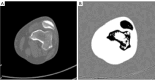
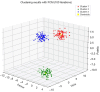
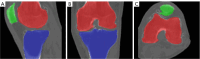
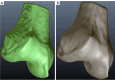
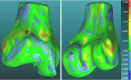
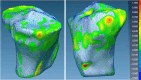
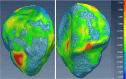
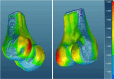
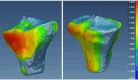
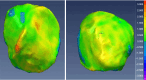
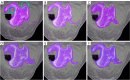
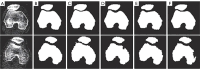
Methods: Our approach integrates the fuzzy C-means (FCM) algorithm with an adaptive region-based active contour model (ACM). Initially, the FCM algorithm assigns membership degrees to each voxel, distinguishing bone regions from surrounding soft tissues based on their likelihood of belonging to specific bone regions. Subsequently, the adaptive region-based ACM utilizes these membership degrees to guide the contour evolution and refine segmentation boundaries. To ensure clinical applicability, we further enhance our method using the marching cubes algorithm to reconstruct a three-dimensional (3D) model. We evaluated the method on six randomly selected knee joints.
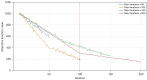
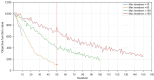
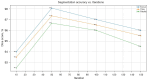
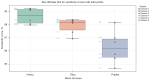
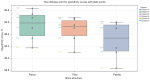
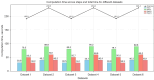
Results: We evaluated the method using quantitative metrics such as the Dice coefficient, sensitivity, specificity, and geometrical assessment. Our method achieved high Dice scores for the femur (98.95%), tibia (98.10%), and patella (97.14%), demonstrating superior accuracy. Remarkably low root mean square distance (RSD) values were obtained for the tibia and femur (0.5±0.14 mm) and patella (0.6±0.13 mm), indicating precise segmentation.
Conclusions: The proposed method offers significant advancements in CAD systems for knee pathologies. Our approach demonstrates superior performance in achieving precise and accurate segmentation of knee bones, providing valuable insights for anatomical analysis, surgical planning, and patient-specific prostheses.
Keywords: Knee bone segmentation; adaptive region based active contour model (adaptive region based ACM); fuzzy-C means (FCM); marching cubes algorithm; three-dimensional reconstruction (3D reconstruction).
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-821/coif). The authors have no conflicts of interest to declare.
Figures





References
-
- Kishore VV, Dosapati UB, Deekshith E, Boyapati K, Pranay S, Kalpana V. CAD Tool for Prediction of Knee Osteoarthritis (KOA). 2024 10th International Conference on Communication and Signal Processing (ICCSP), Melmaruvathur, India, 2024:18-23.
-
- Starmans MP, van der Voort SR, Tovar JMC, Veenland JF, Klein S, Niessen WJ. Radiomics: data mining using quantitative medical image features. Handbook of medical image computing and computer assisted intervention. Academic Press, 2020:429-56.
-
- Walker PS. The Artificial Knee: An Ongoing Evolution. Springer, 2020.
-
- Poliakov A, Pakhaliuk V, Popov VL. Current trends in improving of artificial joints design and technologies for their arthroplasty. Front Mech Eng 2020;6:4.
LinkOut - more resources
Full Text Sources
Miscellaneous
