Revefenacin Area Under the Curve Spirometry in Patients with Moderate to Very Severe COPD
- PMID: 39429809
- PMCID: PMC11491098
- DOI: 10.2147/COPD.S483176
Revefenacin Area Under the Curve Spirometry in Patients with Moderate to Very Severe COPD
Abstract
Purpose: Several lung function endpoints are utilized in clinical trials of inhaled bronchodilators for chronic obstructive pulmonary disease (COPD). Trough forced expiratory volume in 1 second (FEV1) is a commonly reported endpoint in COPD trials and can be complemented by area under the FEV1 vs time curve (FEV1 AUC), which provides information on duration and consistency of bronchodilation over a dosing interval. Revefenacin, a once-daily bronchodilator, significantly improved lung function in patients with COPD when measured by trough FEV1 in two replicate Phase 3 trials. Here, we report an FEV1 AUC substudy using data from these trials.
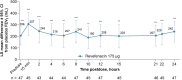
Patients and methods: This post hoc analysis examined substudy data from 12-week replicate Phase 3 trials (NCT02459080/NCT02512510); patients with moderate to very severe COPD were randomized 1:1 to revefenacin 175 μg or placebo once daily. The substudy patients had FEV1 AUC0-2h assessed on Day 1, and those who continued to Day 84 also underwent 24-hour serial spirometry postdose where FEV1 AUC0-2h, AUC0-12h, AUC12-24h, and AUC0-24h were evaluated.
Results: Fifty and 47 patients who received revefenacin and placebo underwent 24-hour serial spirometry; most baseline characteristics were aligned between groups. At Day 84 postdose, revefenacin demonstrated sustained improvements in bronchodilation over 24 hours; differences in least squares mean vs placebo were 282, 220, 205, and 212 mL for FEV1 AUC0-2h, AUC0-12h, AUC12-24h, and AUC0-24h (all P <0.001), respectively.
Conclusion: This substudy analysis supplements previous findings that revefenacin provides sustained bronchodilation over 24 hours. Assessing additional complementary COPD clinical trial endpoints can help clinicians make treatment decisions.
Keywords: Bronchodilators; forced expiratory volume in 1 second; long-acting muscarinic antagonist; outcome measures; spirometry.
© 2024 LeMaster et al.
Conflict of interest statement
Corey J. Witenko is a current employee of Theravance Biopharma US, Inc. and owns stock. Melinda K. Lacy is a current employee of Theravance Biopharma US, Inc. and owns stock. Ann W. Olmsted is a paid consultant for Theravance Biopharma US, Inc. and owns stock. Edmund J. Moran is a current employee of Theravance Biopharma US, Inc. and owns stock. In addition, Edmund J. Moran has a patent US11484531 licensed to Viatris. Donald A. Mahler serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Theravance, Verona, and Viatris and receives royalties from pharmaceutical companies (Elpen Pharmaceutical Company and University of Aberdeen) for the use of baseline dyspnea index/transition dyspnea index. The authors report no other conflicts of interest in this work.
Figures
References
-
- Donohue JF, Kerwin E, Sethi S, et al. Revefenacin, a once-daily, lung-selective, long-acting muscarinic antagonist for nebulized therapy: safety and tolerability results of a 52-week phase 3 trial in moderate to very severe chronic obstructive pulmonary disease. Respir Med. 2019;153:38–43. doi: 10.1016/j.rmed.2019.05.010 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical