New triazole-based hybrids as neurotropic agents
- PMID: 39429923
- PMCID: PMC11487511
- DOI: 10.1039/d4ra06121g
New triazole-based hybrids as neurotropic agents
Abstract
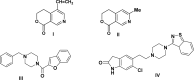
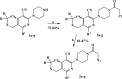
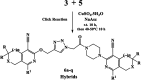
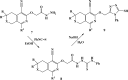
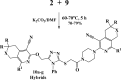
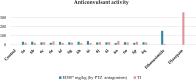
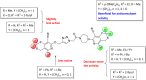
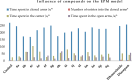
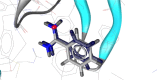
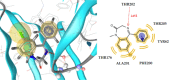
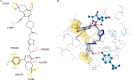
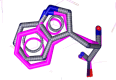
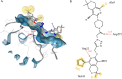
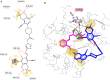
Herein, we describe the synthesis of new hybrids linked to 1,2,3- and 1,2,4-triazole units. Hybrids connected to a 1,2,3-triazole ring were synthesized using the well-known click reaction. The synthesis of the 1,2,4-triazole-based hybrids was carried out using 2-[(4-cyano-1-methyl(2-furyl)-5,6,7,8-tetrahydroisoquinolin-3-yl)oxy]acetohydrazides as starting compounds. The compounds were evaluated for their anticonvulsive activity via antagonism towards pentylenetetrazole (PTZ) - and thiosemicarbazide (TSC)-induced convulsion and maximal electroshock-induced seizure (MES). Furthermore, the most active compounds were studied for their locomotory and anxiolytic activity via the "open field" and elevated plus maze (EPM) assays. Finally, their antidepressant activity was studied via the "forced swim" method. All the hybrids displayed pentylenetetrazole antagonism, ranging from 40% to 80%, while in the TSC model, the most active compounds increased latency of thiosemicarbazide seizures to 1.9-4.65 times compared to that of the control. Some of the tested compounds exhibited a pronounced anxiolytic and antidepressant effect. Docking study demonstrated complete agreement with experimental pharmacological data. It was revealed that the most active compounds have a pyrano[3,4-c]pyridine ring in their structure.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
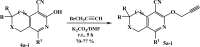



References
-
- Golyala A. Kwan P. Seizure. 2017;44:147–156. - PubMed
-
- Gierbolini J. Giarratano M. Benbadis S. R. Expert Opin. Pharmacother. 2016;17:885–888. - PubMed
-
- Fisher R. S. Acevedo C. Arzimanoglou A. Bogacz A. Cross J. H. Elger C. E. Engel J. Forsgren L. French J. A. Epilepsia. 2014;55:475–482. - PubMed
-
- Voronkova K. V. Golosnaya G. S. Lemeshko I. D. Petrukhin A. S. Epilepsy Paroxysmal Conditions. 2017;9:79–85.
-
- Abdel Salam O. I. Al-Omar M. A. Khalifa N. M. Amr A.-G. Abdallah M. M. Z. Naturforsch., C: J. Biosci. 2013;68:264–268. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

