Prospective Trial on the Pharmacokinetics of Clopidogrel in Hemodialysis Patients
- PMID: 39430173
- PMCID: PMC11489422
- DOI: 10.1016/j.ekir.2024.07.029
Prospective Trial on the Pharmacokinetics of Clopidogrel in Hemodialysis Patients
Abstract
Introduction: Hemodialysis patients (HDPs) exhibit extensive cardiovascular risk. The widely prescribed anti-platelet agent clopidogrel is metabolically activated by cytochrome enzymes, which may be impaired by uremia and chronic low-grade inflammation, typically present in HDPs. We conducted a prospective multicenter study to investigate the pharmacokinetics and pharmacodynamics of clopidogrel in HDPs and healthy volunteers (HVs).
Methods: We enrolled HDPs receiving long-term clopidogrel (75 mg) and pantoprazole treatment (40 mg). Healthy volunteers received a loading dose of 300 mg clopidogrel, followed by 75 mg once daily. Pantoprazole, a substrate and probe drug of CYP2C19, was administered intravenously (40 mg). Plasma concentrations were quantified by mass spectrometry. Pharmacokinetics were calculated, and a population pharmacokinetic model was developed. The primary endpoint was the maximum concentration of clopidogrel's active metabolite. Platelet aggregation was measured using adenosine diphosphate-induced whole-blood aggregometry.
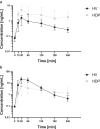
Results: Seventeen HDPs and 16 HVs were included. The maximum concentration of clopidogrel's active metabolite was significantly lower in HDPs compared to HVs (median [interquartile range] 12.2 [4.6-23.4] vs. 24.7 [17.8-36.5] ng/ml, P = 0.02). The maximum concentration ratio of clopidogrel's active metabolite to prodrug was 8.5-fold lower in HDPs, and an 82.7% reduced clopidogrel clearance, including clopidogrel's active metabolite formation, was found using population pharmacokinetic modeling. From previous studies, adenosine diphosphate-induced platelet aggregation at 120 minutes was significantly higher in HDPs than in HVs (median [interquartile range]: 26 U [14 U-43 U] vs. 12 U [11 U-18 U], P = 0.004. Pantoprazole terminal half-life was ∼1.7-fold higher in HDPs compared to HVs.
Conclusion: Our data demonstrate an altered metabolism of clopidogrel in HDPs in the context of lower CYP2C19 activity, with potential implications for other substances metabolized by this enzyme.
Keywords: cardiovascular risk; clopidogrel; cytochrome P450; hemodialysis; pharmacokinetics; platelet aggregation.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures



References
LinkOut - more resources
Full Text Sources

