Imlifidase in Highly Sensitized Kidney Transplant Recipients With a Positive Crossmatch Against a Deceased Donor
- PMID: 39430184
- PMCID: PMC11489446
- DOI: 10.1016/j.ekir.2024.07.024
Imlifidase in Highly Sensitized Kidney Transplant Recipients With a Positive Crossmatch Against a Deceased Donor
Abstract
Introduction: Imlifidase is authorized for desensitization of highly sensitized adult kidney transplant candidates with a positive crossmatch (XM) against a deceased donor. Here, we report on the results for the first 9 patients transplanted in this context who had at least 3 months of follow-up.
Methods: The eligibility criteria were as follows: calculated panel reactive antibodies (cPRA) ³ 98%, ³ 3 years on the waiting list, immunodominant donor-specific antibodies (DSAs) with mean fluorescence intensity (MFI) > 6000 (and < 5000 at 1:10 dilution) and a negative post-imlifidase complement-dependent cytotoxic XM (CDCXM).
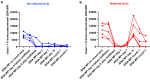
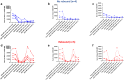
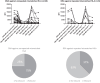
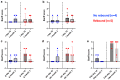
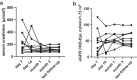
Results: All 9 patients had been on dialysis for an average of 123 ± 41 months, with cPRA at 99% (n = 2) or 100% (n = 7). At transplantation, the mean number of DSAs was 4.3 ± 1.4. The median immunodominant DSA MFI was 9153 (6430-16,980). Flow cytometry XM (FCXM) and CDCXM before imlifidase were positive in 9 and 2 patients, respectively. After 1 injection of imlifidase, all were negative. Patients received polyclonal antibodies, i.v. Igs (IVIg), rituximab, tacrolimus, and mycophenolate. Five patients had a DSA rebound within the first 14 days: 2 had concomitant clinical antibody-mediated rejection (ABMR), 2 had subclinical ABMR, and 1 had isolated positive C4d staining. No ABMR was observed in patients without rebound. Chronic Kidney Disease-Epidemiology Collaboration formula estimated glomerular filtration rate (eGFR) was 56 ± 22 ml/min per 1.73 m2 at the last follow-up (7 ± 2.8 months). No graft loss or death were observed. Four patients developed at least 1 infection.
Conclusion: These real-life data demonstrate that the use of imlifidase to desensitize highly sensitized patients can have an acceptable short-term efficacy and safety profile in selected patients.
Keywords: highly sensitized patients; imlifidase; kidney transplantation; positive crossmatch.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures

References
-
- Jordan S.C., Tyan D., Stablein D., et al. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: report of the NIH IG02 trial. J Am Soc Nephrol. 2004;15:3256–3262. doi: 10.1097/01.ASN.0000145878.92906.9F. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

