Leveraging Patient-Derived Organoids for Personalized Liver Cancer Treatment
- PMID: 39430248
- PMCID: PMC11488587
- DOI: 10.7150/ijbs.96317
Leveraging Patient-Derived Organoids for Personalized Liver Cancer Treatment
Abstract
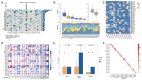
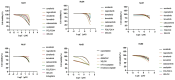
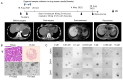
Primary liver cancer (PLC) is a primary cause of cancer-related death worldwide, and novel treatments are needed due to the limited options available for treatment and tumor heterogeneity. 66 surgically removed PLC samples were cultured using the self-developed 2:2 method, and the final success rate for organoid culture was 40.9%. Organoid performance has been evaluated using comprehensive molecular measurements, such as whole-exome and RNA sequencing, as well as anticancer drug testing. Multiple organoids and their corresponding tumor tissues contained several of the same mutations, with all pairs sharing conventional TP53 mutations. Regarding copy number variations and gene expression, significant correlations were observed between the organoids and their corresponding parental tumor tissues. Comparisons at the molecular level provided us with an assessment of organoid-to-tumor concordance, which, in combination with drug sensitivity testing provided direct guidance for treatment selection. Finally, we were able to determine an appropriate pharmacological regimen for a patient with ICC, demonstrating the clinical practicality in tailoring patient-specific drug regimens. Our study provides an organoid culture technology that can cultivate models that retain most of the molecular characteristics of tumors and can be used for drug sensitivity testing, demonstrating the broad potential application of organoid technology in precision medicine for liver cancer treatment.
Keywords: drug screening; organoid; personalized treatment; primary liver cancer.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S. et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Müller M, Bird TG, Nault JC. The landscape of gene mutations in cirrhosis and hepatocellular carcinoma. J Hepatol. 2020;72:990–1002. - PubMed
-
- Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–s16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

