Functional and morphological improvement of significant non-culprit coronary artery stenosis by LDL-C reduction with a PCSK9 antibody: Rationale and design of the randomized FITTER trial
- PMID: 39430462
- PMCID: PMC11489145
- DOI: 10.1016/j.heliyon.2024.e38077
Functional and morphological improvement of significant non-culprit coronary artery stenosis by LDL-C reduction with a PCSK9 antibody: Rationale and design of the randomized FITTER trial
Abstract
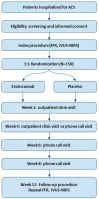
Non-culprit coronary artery lesions are commonly present in patients presenting with an acute coronary syndrome (ACS). Additional stenting of non-culprit lesions in addition to the culprit lesion intends to prevent secondary events caused by these lesions. At the same time, multiple trials have demonstrated the potential of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors in reducing plaque size and changing plaque composition of non-culprit lesions. Whether intensive low-density lipoprotein cholesterol (LDL-C) reduction with PCSK9 inhibitor evolocumab improves non-culprit vessel hemodynamics, reduces the risk of plaque rupture of important non-culprit lesions, and might obviate the need for additional stenting has not been investigated. The "Functional Improvement of non-infarcT related coronary artery stenosis by Extensive LDL-C Reduction with a PCSK9 Antibody" (FITTER) trial is a multi-center, randomized, double-blind, placebo-controlled clinical trial for patients presenting with ACS and multivessel disease (MVD). After treatment of the culprit lesion, fractional flow reserve (FFR) is performed in non-culprit vessels amenable for percutaneous coronary intervention (PCI). Coronary intervention in patients with hemodynamically important non-critical lesions (FFR: 0.67-0.85) is staged after baseline imaging using near-infrared spectroscopy (NIRS) and intravascular ultrasound (IVUS). Eligible patients are randomized and treated for 12 weeks with either evolocumab or placebo, in addition to high-intensity statin therapy. Follow-up angiography with repeat FFR and IVUS-NIRS is scheduled at 12 weeks. Staged PCI is performed at the operator's discretion.The FITTER trial is the first study to evaluate the effect of maximal LDL-C reduction by the PCSK9 inhibitor evolocumab on invasively measured FFR, plaque size, and plaque composition in hemodynamically important non-culprit lesions, during a treatment period of just 12 weeks after an ACS. Currently, all patients have been included (August 2023) and data analysis is ongoing.
Trial registration number: clinicaltrials.gov NCT04141579.
Keywords: Acute coronary syndrome (ACS); Evolocumab; Fractional flow reserve (FFR); Intravascular ultrasound (IVUS); Multivessel disease (MVD); Near-infrared spectroscopy (NIRS); Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Robert-Jan M van Geuns reports financial support and equipment, drugs, or supplies were provided by Amgen Inc. Robert-Jan M van Geuns reports financial support and equipment, drugs, or supplies were provided by 10.13039/100018503Infraredx Inc. Robert-Jan M van Geuns reports financial support was provided by HealthHolland. Pieter C Smits reports a relationship with Abbott Vascular Inc that includes: funding grants and speaking and lecture fees. Pieter C Smits reports a relationship with Shanghai MicroPort Medical Group Co Ltd that includes: funding grants and speaking and lecture fees. Pieter C Smits reports a relationship with SMT that includes: funding grants and speaking and lecture fees. Pieter C Smits reports a relationship with Terumo Medical Corporation that includes: speaking and lecture fees. Robert A Byrne reports a relationship with Abbott Vascular Inc that includes: funding grants. Robert A Byrne reports a relationship with Biosensors that includes: funding grants. Robert A Byrne reports a relationship with 10.13039/100008497Boston Scientific Corporation that includes: funding grants. Robert A Byrne reports a relationship with Translumina GmbH that includes: funding grants. Niels van Royen reports a relationship with BIOTRONIK Inc that includes: funding grants. Niels van Royen reports a relationship with Abbott Vascular Inc that includes: funding grants and speaking and lecture fees. Niels van Royen reports a relationship with Medtronic Inc that includes: funding grants. Niels van Royen reports a relationship with Philips that includes: funding grants. Niels van Royen reports a relationship with Rainmed that includes: speaking and lecture fees. Niels van Royen reports a relationship with Shanghai MicroPort Medical Group Co Ltd that includes: speaking and lecture fees. Niels van Royen reports a relationship with Bayer AG that includes: speaking and lecture fees. Robert-Jan M van Geuns reports a relationship with Abbott Vascular Inc that includes: speaking and lecture fees. Robert-Jan M van Geuns reports a relationship with AstraZeneca that includes: funding grants and speaking and lecture fees. Robert-Jan M van Geuns reports a relationship with Sanofi that includes: funding grants and speaking and lecture fees. Robert-Jan M van Geuns reports a relationship with Amgen Inc that includes: funding grants and speaking and lecture fees. Robert-Jan M van Geuns reports a relationship with 10.13039/100018503Infraredx Inc that includes: funding grants and speaking and lecture fees. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y., Elkind M.S.V., Evenson K.R., Eze-Nliam C., Ferguson J.F., Generoso G., Ho J.E., Kalani R., Khan S.S., Kissela B.M., Knutson K.L., Levine D.A., Lewis T.T., Liu J., Loop M.S., Ma J., Mussolino M.E., Navaneethan S.D., Perak A.M., Poudel R., Rezk-Hanna M., Roth G.A., Schroeder E.B., Shah S.H., Thacker E.L., VanWagner L.B., Virani S.S., Voecks J.H., Wang N.Y., Yaffe K., Martin S.S. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation. 2022;145:e153–e639. doi: 10.1161/cir.0000000000001052. - DOI - PubMed
-
- Balbi M.M., Scarparo P., Tovar M.N., Masdjedi K., Daemen J., Den Dekker W., Ligthart J., Witberg K., Cummins P., Wilschut J., Zijlstra F., Van Mieghem N.M., Diletti R. Culprit lesion detection in patients presenting with non-ST elevation acute coronary syndrome and multivessel disease. Cardiovasc. Revascularization Med. 2022;35:110–118. doi: 10.1016/j.carrev.2021.03.019. - DOI - PubMed
-
- Johnson T.W., Räber L., di Mario C., Bourantas C., Jia H., Mattesini A., Gonzalo N., de la Torre Hernandez J.M., Prati F., Koskinas K., Joner M., Radu M.D., Erlinge D., Regar E., Kunadian V., Maehara A., Byrne R.A., Capodanno D., Akasaka T., Wijns W., Mintz G.S., Guagliumi G. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2019;40:2566–2584. doi: 10.1093/eurheartj/ehz332. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous