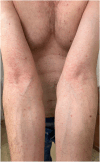
Pityriasis rosea-like drug eruption secondary to deucravacitinib
- PMID: 39430635
- PMCID: PMC11488452
- DOI: 10.1016/j.jdcr.2024.08.019
Pityriasis rosea-like drug eruption secondary to deucravacitinib
Keywords: cutaneous adverse reaction; deucravacitinib; drug rash; medical dermatology; pityriasis rosea.
Conflict of interest statement
Dr Foley has received grant support, and/or served on advisory boards, and/or served as a consultant, and/or has received travel grants, and/or has served as a speaker for or received honoraria from AbbVie, Akaal, Amgen, Arcutis, Argenx, Aslan, AstraZeneca, Boehringer Ingelheim, Botanix, Bristol Myers Squibb, Celgene, Celtaxsys, CSL, Cutanea, Dermira, Evelo, Galderma, GenesisCare, Genentech, GlaxoSmithKline, Hexima, Incyte, Janssen, Kymab, Leo Pharma, Lilly, Mayne Pharma, MedImmune, Melaseq/Geneseq, Merck, Novartis, Pfizer, Regeneron, Reistone, Roche, Sanofi, SunPharma, Takeda, Teva, UCB Pharma, Valeant, and ZaiLab. Drs Punchihewa, Lee, and Tan have no conflicts of interest to declare.
Figures



References
-
- Strober B., Thaci D., Sofen H., et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40–51. doi: 10.1016/j.jaad.2022.08.061. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
