Effectiveness and Safety of a Dermocosmetic Cream as an Adjunct to Adapalene for Mild and Moderate Acne in Indonesia: Results of a Multicenter Randomized Controlled Study
- PMID: 39430644
- PMCID: PMC11490241
- DOI: 10.2147/CCID.S474331
Effectiveness and Safety of a Dermocosmetic Cream as an Adjunct to Adapalene for Mild and Moderate Acne in Indonesia: Results of a Multicenter Randomized Controlled Study
Abstract
Purpose: Acne is a chronic inflammatory skin condition affecting mainly teenagers and adults as well. Guidelines recommend retinoids as a first-line treatment for mild-to-moderate acne. However, dermocosmetics in adjunct could potentially improve efficacy and tolerability. This study was conducted to determine the effectiveness and safety of a dermocosmetic cream containing salicylic acid, lipohydroxy acid, niacinamide, Aqua posae filiformis, procerad and zinc salt in the treatment of mild-to-moderate acne vulgaris in adjunct to different regimens of adapalene compared to adapalene only.
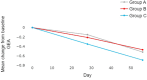
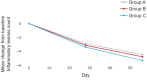
Patients and methods: This randomized, controlled, parallel-group, evaluator-blind study was conducted over 8 weeks on male and female acne subjects at five teaching hospitals in Indonesia. A total of 291 participants were enrolled and divided into three treatment groups: Group A adapalene 0.1% cream nightly - Group B dermocosmetic cream daily + adapalene 0.1% cream every two nights - Group C dermocosmetic cream daily + adapalene 0.1% cream nightly. Clinical evaluations of treatment included scoring on Global Evaluation of Acne (GEA) scale, lesion count (Indonesian Acne Expert Meeting scale), treatment tolerability and treatment satisfaction. Evaluations were performed on Day 28 and Day 56 of treatment.
Results: After 28 and 56 days of treatment, all groups exhibited improvements across the various measures. Data analysis, utilizing Anova for repeated measurements, revealed a statistically significant difference between Groups C and A for reduction of GEA scores (p = 0.038) in favor of Group C. On Day 56, percentages of subjects with GEA Scale improvements of at least 1 grade in comparison with baseline were in Group C (61.7%) followed by Group A (47.9%) and Group B (45.3%). Better treatment tolerance and satisfaction scores were noted in Groups B and C.
Conclusion: Combination of the dermocosmetic cream with adapalene showed higher efficacy, tolerability and satisfaction in comparison to adapalene alone.
Keywords: adjunct to Adapalene; dermocosmetic cream; mild and moderate acne vulgaris.
© 2024 Sitohang et al.
Conflict of interest statement
Delphine Kerob is a full-time employee of La Roche Posay Laboratoire Dermatologique. The authors report no other conflicts of interest in this work.
Figures



References
-
- Sibero HT, Sirajudin A, Anggraini DI. Prevalensi dan gambaran epidemiologi akne vulgaris di Provinsi Lampung. JK Unila. 2019;3(2):308–312. Indonesian.
LinkOut - more resources
Full Text Sources

