A structural, genetic and clinical comparison of CAR-T cells and CAR-NK cells: companions or competitors?
- PMID: 39430751
- PMCID: PMC11486669
- DOI: 10.3389/fimmu.2024.1459818
A structural, genetic and clinical comparison of CAR-T cells and CAR-NK cells: companions or competitors?
Abstract
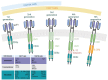
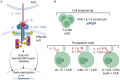
In recent years, following the groundbreaking achievements of chimeric antigen receptor (CAR) T cell therapy in hematological cancers, and advancements in cell engineering technologies, the exploration of other immune cells has garnered significant attention. CAR-Therapy extended beyond T cells to include CAR natural killer (NK) cells and CAR-macrophages, which are firmly established in the clinical trial landscape. Less conventional immune cells are also making their way into the scene, such as CAR mucosal-associated invariant T (MAIT) cells. This progress is advancing precision medicine and facilitating the development of ready-to-use biological treatments. However, in view of the unique features of natural killer cells, adoptive NK cell immunotherapy has emerged as a universal, allogenic, "off-the shelf" therapeutic strategy. CAR-NK cytotoxic cells present targeted tumor specificity but seem to be devoid of the side effects associated with CAR-T cells. CAR-NK cells appear to be potentially promising candidates for cancer immunotherapy. However, their application is hindered by significant challenges, particularly the limited persistence of CAR-NK cells in the body, which poses a hurdle to their sustained effectiveness in treating cancer. Based upon the foregoing, this review discusses the current status and applications of both CAR-T cells and CAR-NK cells in hematological cancers, and provides a comparative analysis of the structure, genetics, and clinical outcomes between these two types of genetically modified immune cells.
Keywords: CAR-NK cells; CAR-T cells; Cytokines; Hematological malignancies; Off-the-shelf Immunotherapies.
Copyright © 2024 Andrea, Chiron, Sarrabayrouse, Bessoles and Hacein-Bey-Abina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

