Hemopexin alleviates sterile inflammation in ischemia-reperfusion-induced lung injury
- PMID: 39430764
- PMCID: PMC11487521
- DOI: 10.3389/fimmu.2024.1451577
Hemopexin alleviates sterile inflammation in ischemia-reperfusion-induced lung injury
Abstract
Introduction: Pulmonary ischemia-reperfusion (IR) injury (IRI) plays a significant role in various lung disorders and is a key factor in the development of primary graft dysfunction following lung transplantation. Hemopexin (Hx) is the major serum scavenger protein for heme, which is a prooxidant and pro-inflammatory compound. In the current study, we hypothesized that Hx could confer beneficial effects in sterile inflammation induced by IR-mediated lung injury.
Methods: To examine this hypothesis, we administered Hx in an experimental mouse model of unilateral lung IRI.
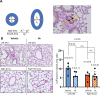
Results: Our results demonstrate that treatment with Hx alleviated histopathological signs of inflammation in ischemic lungs, as evidenced by a reduction in the number of infiltrating neutrophils and decreased levels of perivascular edema. In addition, thrombotic vaso-occlusion in pulmonary blood vessels of IRI lungs was reduced by Hx. Immunohistochemical analysis revealed that Hx inhibited the up-regulation of heme oxygenase-1, an enzyme highly induced by heme, in ischemic lungs. Finally, Hx administration caused a decrease in the levels of circulating B- and CD8+ T-lymphocytes in the peripheral blood of mice with pulmonary IRI.
Conclusion: These findings suggest that the serum heme scavenger protein Hx holds therapeutic promise in alleviating lung IRI-mediated sterile inflammation. Thus, Hx may represent a preemptive therapeutic approach in IR-related lung disorders such as primary graft dysfunction in lung transplantation.
Keywords: heme; hemopexin; ischemia-reperfusion injury; lung; sterile inflammation.
Copyright © 2024 Nakagiri, Köhler, Janciauskiene, Neubert, Knöfel, Pradhan, Ruhparwar, Ius and Immenschuh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

