Sex- and time-dependent role of insulin regulated aminopeptidase in lipopolysaccharide-induced inflammation
- PMID: 39430768
- PMCID: PMC11486674
- DOI: 10.3389/fimmu.2024.1466692
Sex- and time-dependent role of insulin regulated aminopeptidase in lipopolysaccharide-induced inflammation
Abstract
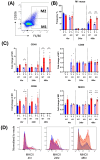
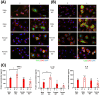
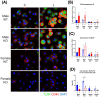
The enzyme, insulin regulated aminopeptidase (IRAP), is expressed in multiple immune cells such as macrophages, dendritic cells and T cells, where it plays a role in regulating the innate and adaptive immune response. There is a genetic association between IRAP and survival outcomes in patients with septic shock where a variant of its gene was found to be associated with increased 28-day mortality. This study investigated the role for IRAP in a lipopolysaccharide (LPS)-induced inflammatory response which is thought to model facets of the systemic inflammation observed in the early stages of human gram-negative sepsis. The frequencies and activation of splenic immune cell populations were investigated in the IRAP knockout (KO) mice compared to the wildtype controls over a period of 4-, 24-, or 48-hours following LPS stimulation. Dendritic cells isolated from the spleen of female IRAP KO mice, displayed significant increases in the activation markers CD40, CD86 and MHCII at 24 hours after LPS induction. A modest heightened pro-inflammatory response to LPS was observed with increased expression of activation marker CD40 in M1 macrophages from male IRAP knockout mice. Observations in vitro in bone marrow-derived macrophages (BMDM) revealed a heightened pro-inflammatory response to LPS with significant increases in the expression of CD40 in IRAP deficient cells compared with BMDM from WT mice. The heightened LPS-induced response was associated with increased pro-inflammatory cytokine secretion in these BMDM cells. A genotype difference was also detected in the BMDM from female mice displaying suppression of the LPS-induced increases in the activation markers CD40, CD86, CD80 and MHCII in IRAP deficient cells. Thus, this study suggests that IRAP plays specific time- and sex-dependent roles in the LPS-induced inflammatory response in dendritic cells and macrophages.
Keywords: IRAP; LPS; gram-negative sepsis; inflammation; insulin regulated aminopeptidase; lipopolysaccharide.
Copyright © 2024 Vear, Chakraborty, Fahimi, Ferens, Widdop, Samuel, Gaspari, van Endert and Chai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

