Empagliflozin Use Is Associated With Lower Risk of All-Cause Mortality, Hospitalization for Heart Failure, and End-Stage Renal Disease Compared to DPP-4i in Nordic Type 2 Diabetes Patients: Results From the EMPRISE (Empagliflozin Comparative Effectiveness and Safety) Study
- PMID: 39430801
- PMCID: PMC11490347
- DOI: 10.1155/2024/6142211
Empagliflozin Use Is Associated With Lower Risk of All-Cause Mortality, Hospitalization for Heart Failure, and End-Stage Renal Disease Compared to DPP-4i in Nordic Type 2 Diabetes Patients: Results From the EMPRISE (Empagliflozin Comparative Effectiveness and Safety) Study
Abstract
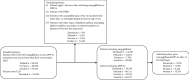
Objective: To evaluate the effectiveness of empagliflozin in reducing all-cause mortality (ACM), hospitalization for heart failure (HHF), myocardial infarction (MI), stroke, cardiovascular mortality (CVM), and end-stage renal disease (ESRD) in routine clinical practice in the Nordic countries of the Empagliflozin Comparative Effectiveness and Safety (EMPRISE) study. Methods: This noninterventional, multicountry cohort study used secondary data from four Nordic countries (Denmark, Sweden, Finland, and Norway). Propensity score (PS) matched (1:1) adults with type 2 diabetes (T2D) initiating empagliflozin (a sodium-glucose cotransporter-2 inhibitor) during 2014-2018 who were compared to those initiating a dipeptidyl peptidase-4 inhibitor (DPP-4i). Cox proportional hazards regression modelling was used to assess the risk for ACM, HHF, MI, stroke, CVM, and ESRD. Meta-analyses were conducted and hazard ratios (HRs) with 95% confidence intervals (CIs) from random-effects models were calculated. Results: A total of 43,695 pairs of PS-matched patients were identified. Patients initiating empagliflozin exhibited a 49% significantly lower risk of ACM (HR: 0.51, 95% CI 0.40-0.64) compared to DPP-4i. Additionally, empagliflozin was associated with a 36% significantly lower risk of HHF (HR: 0.64, 95% CI 0.46-0.89), a 52% significantly lower risk of CVM (HR: 0.48, 95% CI 0.37-0.63), and a 66% significantly lower risk of ESRD (HR: 0.34, 95% CI 0.15-0.77) compared to DPP-4i. No significant differences were observed in the risk of stroke and MI between patients initiating empagliflozin compared with those initiating a DPP-4i. Results were generally consistent for subgroups (with/without pre-existing CV disease or congestive heart failure) and in sensitivity analyses. Conclusion: Empagliflozin initiation was associated with a significantly reduced risk of ACM, HHF, CVM, and ESRD compared with initiation of DPP-4i in patients with T2D when examining routine clinical practice data from Nordic countries.
Keywords: cardiovascular diseases; comparative effectiveness; dipeptidyl peptidase-4 inhibitors; empagliflozin; end-stage renal disease; heart failure; sodium-glucose cotransporter-2 inhibitors; type 2 diabetes mellitus.
Copyright © 2024 Gisle Langslet et al.
Conflict of interest statement
Dorte Vistisen has received research grants from Bayer A/S, Sanofi, Novo Nordisk A/S, and Boehringer Ingelheim. She holds shares in Novo Nordisk A/S. Bendix Carstensen declares no conflicts of interest. Sigrun Halvorsen has received speaker fees from Sanofi, Novartis, Boehringer Ingelheim, Bayer, Pfizer, and Bristol-Myers Squibb. Gisle Langslet has received consulting/lecture fees from Sanofi and Boehringer Ingelheim. Thomas Nyström has received unrestricted grants from AstraZeneca and Novo Nordisk and has been a national adviser of Abbot, Amgen, Novo Nordisk, Sanofi-Aventis, Eli Lilly, MSD, and Boehringer Ingelheim. Leo Niskanen has received speaker honoraria from Amgen, Boehringer Ingelheim, Novo Nordisk, Sanofi, MSD, and Astra Zeneca; research support from Novo Nordisk to the hospital; and has participated in the scientific advisory boards of Amgen, Boehringer Ingelheim, AstraZeneca, MSD, and Novo Nordisk. Paula Casajust is an employee of TFS Health Science. Giorgi Tskhvarashvili, Fabian Hoti, and Riho Klement are/were employees of IQVIA contracted by Boehringer Ingelheim to conduct the meta-analyses, interpret the results, review, and revise the manuscript. Christina Shay, Soulmaz Fazeli Farsani, Kristina Karlsdotter, Mikko Tuovinen, Anne Pernille Ofstad, and Maria Lajer were employees of Boehringer Ingelheim at the time of manuscript development. Lisette Koeneman is an employee of Eli Lilly and Company and owns stock in Eli Lilly and Company. Emilie Toresson Grip is an employee of Quantify Research that was contracted to conduct the country-specific studies in Finland, Norway, and Sweden.
Figures








References
-
- Federation I. D. IDF diabetes atlas, tenth 2021. http://diabetesatlas.org/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

