Early-life obesogenic environment integrates immunometabolic and epigenetic signatures governing neuroinflammation
- PMID: 39430879
- PMCID: PMC11490928
- DOI: 10.1016/j.bbih.2024.100879
Early-life obesogenic environment integrates immunometabolic and epigenetic signatures governing neuroinflammation
Abstract
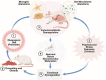
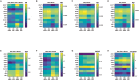
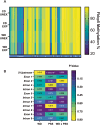
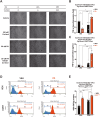
Childhood overweight/obesity is associated with stress-related psychopathology, yet the pathways connecting childhood obesity to stress susceptibility are poorly understood. We employed a systems biology approach with 62 adolescent Lewis rats fed a Western-like high-saturated fat diet (WD, 41% kcal from fat) or a control diet (CD, 13% kcal from fat). A subset of rats underwent a 31-day model of predator exposures and social instability (PSS). Effects were assessed using behavioral tests, DTI (diffusion tensor imaging), NODDI (neurite orientation dispersion and density imaging), 16S rRNA gene sequencing for gut microbiome profiling, hippocampal microglia analysis, and targeted gene methylation. Parallel experiments on human microglia cells (HMC3) examined how palmitic acid influences cortisol-related inflammatory responses. Rats exposed to WD and PSS exhibited deficits in sociability, increased fear/anxiety-like behaviors, food consumption, and body weight. WD/PSS altered hippocampal microstructure (subiculum, CA1, dentate gyrus), and microbiome analysis showed a reduced abundance of members of the phylum Firmicutes. WD/PSS synergistically promoted neuroinflammatory changes in hippocampal microglia, linked with microbiome shifts and altered Fkbp5 expression/methylation. In HMC3, palmitate disrupted cortisol responses, affecting morphology, phagocytic markers, and cytokine release, partially mediated by FKBP5. This study identifies gene-environment interactions that influence microglia biology and may contribute to the connection between childhood obesity and stress-related psychopathology later in life.
Keywords: Adolescence; Anxiety; FKBP5; Microbiome; Microglia; NODDI; Neuroinflammation; Obesity.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest related to this work.
Figures







References
-
- Adamec R.E., Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiol. Behav. 1993;54:101–109. - PubMed
-
- Adamec R., Strasser K., Blundell J., Burton P., McKay D.W. Protein synthesis and the mechanisms of lasting change in anxiety induced by severe stress. Behav. Brain Res. 2006;167:270–286. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

