Design, synthesis, and structure-activity relationship studies of 6 H-benzo[ b]indeno[1,2- d]thiophen-6-one derivatives as DYRK1A/CLK1/CLK4/haspin inhibitors
- PMID: 39430953
- PMCID: PMC11487425
- DOI: 10.1039/d4md00537f
Design, synthesis, and structure-activity relationship studies of 6 H-benzo[ b]indeno[1,2- d]thiophen-6-one derivatives as DYRK1A/CLK1/CLK4/haspin inhibitors
Abstract
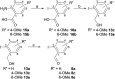
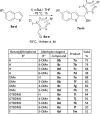
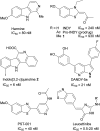
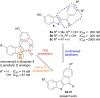
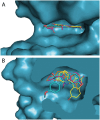
A series of sulfur-containing tetracycles was designed and evaluated for their ability to inhibit protein kinase DYRK1A, a target known to have several potential therapeutic applications including cancers, Down syndrome or Alzheimer's disease. Our medicinal chemistry strategy relied on the design of new compounds using ring contraction/isosteric replacement and constrained analogy of known DYRK1A inhibitors, thus resulting in their DYRK1A inhibitory activity enhancement. Whereas a good inhibitory effect of targeted DYRK1A protein was observed for 5-hydroxy compounds 4i-k (IC50 = 35-116 nM) and the 5-methoxy derivative 4e (IC50 = 52 nM), a fairly good selectivity towards its known DYRK1B off-target was observed for 4k. In addition, the most active compound 4k, having an ATP-competitive mechanism of action, proved to be also a potent inhibitor of CLK1/CLK4 (IC50 = 20 and 26 nM) and, to a lesser extent, of haspin (IC50 = 76 nM) kinases. In silico docking studies within the DYRK1A, CLK1/CLK4 and haspin ATP binding sites were carried out to understand the interactions of our tetracyclic derivatives 4 with these targets. Antiproliferative activities on U87/U373 glioblastoma cell lines of the most potent compound 4k showed a moderate effect (IC50 values between 33 and 46 μM). Microsomal stabilities of the designed compounds 4a-m were also investigated, showing great disparities, depending on benzo[b]thiophene ring 5-substitution.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Druker B. J. Tamura S. Buchdunger E. Ohno S. Segal G. M. Fanning S. Zimmermann J. Lydon N. B. Nat. Med. 1996;2:561–566. - PubMed
-
- Druker B. J. Guilhot F. O'Brien S. G. Gathmann I. Kantarjian H. Gattermann N. Deininger M. W. N. Silver R. T. Goldman J. M. Stone R. M. Cervantes F. Hochhaus A. Powell B. L. Gabrilove J. L. Rousselot P. Reiffers J. Cornelissen J. J. Hughes T. Agis H. Fischer T. Verhoef G. Shepherd J. Saglio G. Gratwohl A. Nielsen J. L. Radich J. P. Simonsson B. Taylor K. Baccarani M. So C. Letvak L. Larson R. A. N. Engl. J. Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. - DOI - PubMed
-
- Demetri G. D. von Mehren M. Blanke C. D. Van den Abbeele A. D. Eisenberg B. Roberts P. J. Heinrich M. C. Tuveson D. A. Singer S. Janicek M. Fletcher J. A. Silverman S. G. Silberman S. L. Capdeville R. Kiese B. Peng B. Dimitrijevic S. Druker B. J. Corless C. Fletcher C. D. M. Joensuu H. N. Engl. J. Med. 2002;347:472–480. - PubMed
LinkOut - more resources
Full Text Sources

