Preclinical pharmacology of patient-derived extracellular vesicles for the intraoperative imaging of tumors
- PMID: 39431003
- PMCID: PMC11488097
- DOI: 10.7150/thno.98671
Preclinical pharmacology of patient-derived extracellular vesicles for the intraoperative imaging of tumors
Abstract
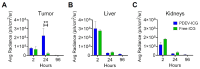
Extracellular vesicles (EVs) derived from the plasma of oncological patients exhibit significant tumor-targeting properties, unlike those from healthy individuals. We have previously shown the feasibility of formulating the near-infrared (NIR) fluorescent dye indocyanine green (ICG) with patient-derived extracellular vesicles (PDEVs) for selective delivery to neoplastic tissue. This staining protocol holds promise for clinical application in intraoperative tumor margin imaging, enabling precise neoplastic tissue resection. To this end, we propose the ONCOGREEN protocol, involving PDEV isolation, ICG loading, and reinfusion into the same patients. Methods: By in vivo studies on mice, we outlined key pharmacological parameters of PDEVs-ICG for intraoperative tumor imaging, PDEV biodistribution kinetics, and potential treatment-related toxicological effects. Additionally, we established a plasmapheresis-based protocol for isolating autologous PDEVs, ensuring the necessary large-scale dosage for human treatment. A potential lyophilization-based preservation method was also explored to facilitate the storage and transport of PDEVs. Results: The study identified the effective dose of PDEVs-ICG necessary for clear intraoperative tumor margin imaging. The biodistribution kinetics of PDEVs showed favorable targeting to neoplastic tissues, without off-target distribution. Toxicological assessments revealed no significant adverse effects associated with the treatment. The plasmapheresis-based isolation protocol successfully yielded a sufficient quantity of autologous PDEVs, and the lyophilization preservation method maintained the functional integrity of PDEVs for subsequent clinical application. Conclusions: Our research lays the groundwork for the direct clinical application of autologous PDEVs, initially focusing on intraoperative imaging. Utilizing autologous PDEVs has the potential to accelerate the integration of EVs as a targeted delivery tool for anti-neoplastic agents to cancerous tissue. This approach promises to enhance the precision of neoplastic tissue resection and improve overall surgical outcomes for oncological patients.
Keywords: Bench-to-bedside Translation; EV Biodistribution Kinetics; Intraoperative Imaging; Toxicology.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

