Preoperative PET imaging and fluorescence-guided surgery of human glioblastoma using dual-labeled antibody targeting ETA receptors in a preclinical mouse model: A theranostic approach
- PMID: 39431005
- PMCID: PMC11488107
- DOI: 10.7150/thno.98163
Preoperative PET imaging and fluorescence-guided surgery of human glioblastoma using dual-labeled antibody targeting ETA receptors in a preclinical mouse model: A theranostic approach
Abstract
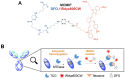
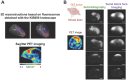
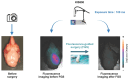
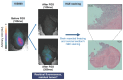
Rationale: Glioblastoma (GBM) poses significant challenges regarding complete tumor removal due to its heterogeneity and invasiveness, emphasizing the need for effective therapeutic options. In the last two decades, fluorescence-guided surgery (FGS), employing fluorophores such as 5-aminolevulinic acid (5-ALA) to enhance tumor delineation, has gained attraction among neurosurgeons. However, some low-grade tumors do not show any accumulation of the tracers, and the lack of patient stratification represents an important limitation. Since 2000, endothelin axis has been extensively investigated for its role in cancer progression. More specifically, our team has identified endothelin A receptors (ETA), overexpressed in glioblastoma cancer stem cells, as a target of interest for GBM imaging. This study aims to evaluate the efficacy of a novel preclinical bimodal imaging agent, [89Zr]Zr-axiRA63-MOMIP, as a theranostic approach to: i) detect ETA + cells in an orthotopic model of human GBM, ii) achieve complete tumoral resection. Methods: Monomolecular multimodal imaging platform (MOMIP) - containing both a fluorophore (IRDye800CW) and a chelator for a positron-emitting radiometal (desferroxamine B, DFO) - was conjugated to the axiRA63 antibody targeting ETA receptors, overexpressed on the surface of GBM stem cells. Mice bearing orthotopic human GBM were imaged 48 h post injection of [89Zr]Zr-axiRA63-MOMIP via positron emission tomography (PET) and optical imaging. Subsequently, post-mortem proof-of-concept FGS was implemented as well as ex vivo analyses (H&E staining, autoradiography, serial block face imaging) on brains with resected or unresected tumor to assess the correlation between PET and fluorescence signals. Results: PET imaging of [89Zr]Zr-axiRA63-MOMIP enabled a clear detection of ETA + cells in an orthotopic model of human GBM. Intraoperative optical imaging allowed a near-complete tumor resection together with the visualization of a weak fluorescence signal, after a prolonged exposure time, that was attributed to residual tumor cells via H&E staining. Besides, a qualitative correlation between the signals of both modalities was observed. Conclusions: The use of [89Zr]Zr-axiRA63-MOMIP provides an effective theranostic approach to detect and treat GBM by surgery in a preclinical mouse model. Thanks to the high correlation between PET and fluorescence signal allowing patients stratification, this bimodal agent should have a great potential for clinical translation and should present a significant advantage over non-targeted fluorophores already used in the clinic.
Keywords: Bimodal Imaging; Endothelin A (ETA); Fluorescence-Guided surgery; Glioblastoma; ImmunoPET.
© The author(s).
Conflict of interest statement
Competing Interests: DB and AH are scientific cofounders and hold equity in Skymab Biotherapeutics.
Figures


References
-
- Wykes V, Zisakis A, Irimia M, Ughratdar I, Sawlani V, Watts C. Importance and evidence of extent of resection in glioblastoma. J Neurol Surg A Cent Eur Neurosurg. 2021;82:075–86. - PubMed
-
- Fernandes C, Costa A, Osório L, Lago RC, Linhares P, Carvalho B, Current standards of care in glioblastoma therapy. In: De Vleeschouwer S, Ed. Glioblastoma. Brisbane (AU): Codon Publications. 2017. pp. 197–241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

