Single-cell profiling of brain pericyte heterogeneity following ischemic stroke unveils distinct pericyte subtype-targeted neural reprogramming potential and its underlying mechanisms
- PMID: 39431007
- PMCID: PMC11488099
- DOI: 10.7150/thno.97165
Single-cell profiling of brain pericyte heterogeneity following ischemic stroke unveils distinct pericyte subtype-targeted neural reprogramming potential and its underlying mechanisms
Abstract
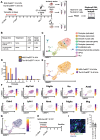
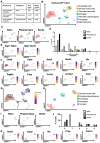
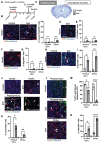
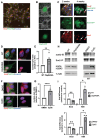
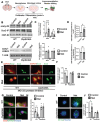
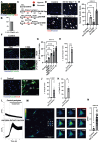
Rationale: Brain pericytes can acquire multipotency to produce multi-lineage cells following injury. However, pericytes are a heterogenous population and it remains unknown whether there are different potencies from different subsets of pericytes in response to injury. Methods: We used an ischemic stroke model combined with pericyte lineage tracing animal models to investigate brain pericyte heterogeneity under both naïve and brain injury conditions via single-cell RNA-sequencing and immunohistochemistry analysis. In addition, we developed an NG2+ pericyte neural reprogramming culture model from both murine and humans to unveil the role of energy sensor, AMP-dependent kinase (AMPK), activity in modulating the reprogramming/differentiation process to convert pericytes to functional neurons by targeting a Ser 436 phosphorylation on CREB-binding protein (CBP), a histone acetyltransferase. Results: We showed that two distinct pericyte subpopulations, marked by NG2+ and Tbx18+, had different potency following brain injury. NG2+ pericytes expressed dominant neural reprogramming potential to produce newborn neurons, while Tbx18+ pericytes displayed dominant multipotency to produce endothelial cells, fibroblasts, and microglia following ischemic stroke. In addition, we discovered that AMPK modulators facilitated pericyte-to-neuron conversion by modulating Ser436 phosphorylation status of CBP, to coordinate an acetylation shift between Sox2 and histone H2B, and to regulate Sox2 nuclear-cytoplasmic trafficking during the reprogramming/differentiation process. Finally, we showed that sequential treatment of compound C (CpdC) and metformin, AMPK inhibitor and activator respectively, robustly facilitated the conversion of human pericytes into functional neurons. Conclusion: We revealed that two distinct subtypes of pericytes possess different reprogramming potencies in response to physical and ischemic injuries. We also developed a genomic integration-free methodology to reprogram human pericytes into functional neurons by targeting NG2+ pericytes.
Keywords: CBP S436 phosphorylation; Sox2; acetylation; cellular reprogramming; focal ischemic stroke; histone 2B; induced neural stem cells; neuronal differentiation; pericytes.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Yamanishi E, Takahashi M, Saga Y, Osumi N. Penetration and differentiation of cephalic neural crest-derived cells in the developing mouse telencephalon. Dev Growth Differ. 2012;54:785–800. - PubMed
-
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

