PET imaging of microglia in Alzheimer's disease using copper-64 labeled TREM2 antibodies
- PMID: 39431020
- PMCID: PMC11488106
- DOI: 10.7150/thno.97149
PET imaging of microglia in Alzheimer's disease using copper-64 labeled TREM2 antibodies
Abstract
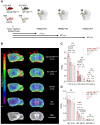
Triggering receptor expressed on myeloid cells 2 (TREM2) plays an essential role in microglia activation and is being investigated as a potential therapeutic target for modulation of microglia in several neurological diseases. In this study, we present the development and preclinical evaluation of 64Cu-labeled antibody-based PET radiotracers as tools for non-invasive assessment of TREM2 expression. Furthermore, we tested the potential of an antibody transport vehicle (ATV) that binds human transferrin receptor to facilitate transcytosis of TREM2 antibody-based radiotracers to the CNS and improve target engagement. Methods: A TREM2 antibody with an engineered transport vehicle (ATV:4D9) and without (4D9) were covalently modified with pNCS-benzyl-NODAGA and labeled with copper-64. Potency, stability, and specificity were assessed in vitro followed by in vivo PET imaging at the early 2 h, intermediate 20 h, and late imaging time points 40 h post-injection using a human transferrin receptor (hTfR) expressing model for amyloidogenesis (5xFAD;TfRmu/hu) or wild-type mice (WT;TfRmu/hu), and hTfR negative controls. Organs of interest were isolated to determine biodistribution by ex vivo autoradiography. Cell sorting after in vivo tracer injection was used to demonstrate cellular specificity for microglia and to validate TREM2 PET results in an independent mouse model for amyloidogenesis (AppSAA;TfRmu/hu). For translation to human imaging, a human TREM2 antibody (14D3) was radiolabeled and used for in vitro autoradiography on human brain sections. Results: The 64Cu-labeled antibodies were obtained in high radiochemical purity (RCP), radiochemical yield (RCY), and specific activity. Antibody modification did not impact TREM2 binding. ATV:4D9 binding proved to be specific, and the tracer stability was maintained over 48 h. The uptake of [64Cu]Cu-NODAGA-ATV:4D9 in the brains of hTfR expressing mice was up to 4.6-fold higher than [64Cu]Cu-NODAGA-4D9 in mice without hTfR. TREM2 PET revealed elevated uptake in the cortex of 5xFAD mice compared to wild-type, which was validated by autoradiography. PET-to-biodistribution correlation revealed that elevated radiotracer uptake in brains of 5xFAD;TfRmu/hu mice was driven by microglia-rich cortical and hippocampal brain regions. Radiolabeled ATV:4D9 was selectively enriched in microglia and cellular uptake explained PET signal enhancement in AppSAA;TfRmu/hu mice. Human autoradiography showed elevated TREM2 tracer binding in the cortex of patients with Alzheimer's disease. Conclusion: [64Cu]Cu-NODAGA-ATV:4D9 has potential for non-invasive assessment of TREM2 as a surrogate marker for microglia activation in vivo. ATV engineering for hTfR binding and transcytosis overcomes the blood-brain barrier restriction for antibody-based PET radiotracers. TREM2 PET might be a versatile tool for many applications beyond Alzheimer's disease, such as glioma and chronic inflammatory diseases.
Keywords: ATV:4D9; PET; TREM2; copper-64; microglia.
© The author(s).
Conflict of interest statement
Competing Interests: G.C.P., D.X., and K.M.M. are full-time employees and shareholders of Denali Therapeutics. The other authors have declared that no competing interest exists.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

