Use of NIR in COVID-19 Screening: Proof of Principles for Future Application
- PMID: 39431082
- PMCID: PMC11483380
- DOI: 10.1021/acsomega.4c06092
Use of NIR in COVID-19 Screening: Proof of Principles for Future Application
Abstract
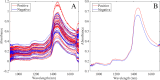
The COVID-19 pandemic that affected the world between 2019 and 2022 showed the need for new tools to be tested and developed to be applied in global emergencies. Although standard diagnostic tools exist, such as the reverse-transcription polymerase chain reaction (RT-PCR), these tools have shown severe limitations when mass application is required. Consequently, a pressing need remains to develop a rapid and efficient screening test to deliver reliable results. In this context, near-infrared spectroscopy (NIRS) is a fast and noninvasive vibrational technique capable of identifying the chemical composition of biofluids. This study aimed to develop a rapid NIRS testing methodology to identify individuals with COVID-19 through the spectral analysis of swabs collected from the oral cavity. Swab samples from 67 hospitalized individuals were analyzed using NIR equipment. The spectra were preprocessed, outliers were removed, and classification models were constructed using partial least-squares for discriminant analysis (PLS-DA). Two models were developed: one with all the original variables and another with a limited number of variables selected using ordered predictors selection (OPS-DA). The OPS-DA model effectively reduced the number of redundant variables, thereby improving the diagnostic metrics. The model achieved a sensitivity of 92%, a specificity of 100%, an accuracy of 95%, and an AUROC of 94% for positive samples. These preliminary results suggest that NIRS could be a potential tool for future clinical application. A fast methodology for COVID-19 detection would facilitate medical diagnoses and laboratory routines, helping to ensure appropriate treatment.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Chinazzi M.; Davis J. T.; Ajelli M.; Gioannini C.; Litvinova M.; Merler S.; Pastore y Piontti A.; Mu K.; Rossi L.; Sun K.; et al. The Effect of Travel Restrictions on the Spread of the 2019 Novel Coronavirus (COVID-19) Outbreak. Science 2020, 368 (6489), 395–400. 10.1126/science.aba9757. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
