Synthesis of a mixed-linker Ce-UiO-67 metal-organic framework
- PMID: 39431242
- PMCID: PMC11487926
- DOI: 10.1039/d4lf00278d
Synthesis of a mixed-linker Ce-UiO-67 metal-organic framework
Abstract
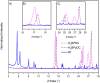
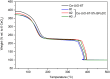
Ce-based metal-organic frameworks (MOFs) have recently gained scientific interest, since Ce is the most abundant rare-earth element in the Earth's crust and since their synthesis has some advantages, including first of all their redox activity, the high porosity of these crystalline materials, and Ce availability. In particular, Ce(iv)-based MOFs, such as Ce-UiO-66 and Ce-UiO-67, are synthesised under mild conditions. For most applications, the presence of functional groups in the frameworks is needed; in this context, linkers containing N-functionalities have been highlighted, as they allow for the incorporation of a large variety of metal cations. In order to insert N-functionalities for the sake of successive metal-functionalization of materials as Ce-UiO-67, we have successfully synthesised a mixed-linker version of this MOF, by incorporating 2,2'-bipyridine-5,5'-dicarboxylic acid together with the conventional biphenyl-4,4'-dicarboxylic acid linker; we have worked on a reproducible and upscalable procedure using benzoic acid as the modulator, without altering the original framework topology. Mixed-linker Ce-UiO-67 MOFs exhibit good thermal stability, high Brunauer-Emmett-Teller (BET) SSAs and microporosity. The pristine samples are highly stable if stored in a desiccator, as demonstrated by the preservation of their high crystallinity for at least 18 months.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures



References
-
- Hoskins B. F. Robson R. J. Am. Chem. Soc. 1989;111:5962–5964. doi: 10.1021/ja00197a079. - DOI
-
- Li H. Eddaoudi M. O'Keeffe M. et al. . Nature. 1999;402:276–279. doi: 10.1038/46248. - DOI
-
- https://www.usgs.gov/centers/national-minerals-information-center/rare-e..., (accessed 5 April 2024)
-
- https://www.euchems.eu/euchems-periodic-table/, (accessed 6 September 2024)
LinkOut - more resources
Full Text Sources
