Droplet-based fluorescence anisotropy insulin immunoassay
- PMID: 39431529
- PMCID: PMC11492383
- DOI: 10.1039/d4ay01511h
Droplet-based fluorescence anisotropy insulin immunoassay
Abstract
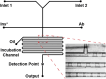
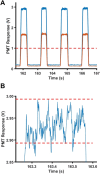
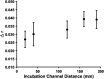
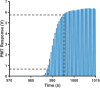
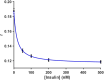
Over the last several decades, multiple microfluidic platforms have been used for measurement of hormone secretion from islets of Langerhans. Most have used continuous flow systems where mixing of hormones with assay reagents is governed by diffusion, leading to long mixing times, especially for biomolecules like peptides and proteins which have large diffusion coefficients. Consequently, dispersion of rapidly changing signals can occur, reducing temporal resolution. Droplet microfluidic systems can be used to capture reagents into individual reactors, limiting dispersion and improving temporal resolution. In this study, we integrated a fluorescence anisotropy (FA) immunoassay (IA) for insulin into a droplet microfluidic system. Insulin IA reagents were mixed online with insulin and captured quickly into droplets prior to passing through a 200 mm incubation channel. Double etching of the glass device was used to increase the depth of the incubation channel compared to the IA channels to maintain proper flow of reagents. The droplet system produced highly precise FA results with relative standard deviations < 2% at all insulin concentrations tested, whereas the absolute fluorescence intensity precisions ranged between 5 and 6%. A limit of detection of 3 nM for insulin was obtained, similar to those found in conventional flow systems. The advantage of the system was in the increased temporal resolution using the droplet system where a 9.8 ± 2.6 s response time was obtained, faster than previously reported continuous flow systems. The improved temporal resolution aligns with continued efforts to resolve rapid signaling events in pancreatic islet biology.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
-
- Saeedi P. Petersohn I. Salpea P. Malanda B. Karuranga S. Unwin N. Colagiuri S. Guariguata L. Motala A. A. Ogurtsova K. Shaw J. E. Bright D. Williams R. Committee on behalf of the IDA Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

