Development and validation of a chronic kidney disease progression model using patient-level simulations
- PMID: 39431558
- PMCID: PMC11494709
- DOI: 10.1080/0886022X.2024.2406402
Development and validation of a chronic kidney disease progression model using patient-level simulations
Abstract
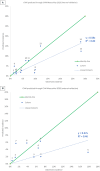
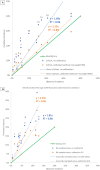
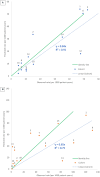
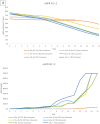
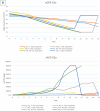
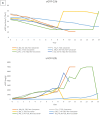
Chronic disease progression models are available for several highly prevalent conditions. For chronic kidney disease (CKD), the scope of existing progression models is limited to the risk of kidney failure and major cardiovascular (CV) events. The aim of this project was to develop a comprehensive CKD progression model (CKD-PM) that simulates the risk of CKD progression and a broad range of complications in patients with CKD. A series of literature reviews informed the selection of risk factors and identified existing risk equations/algorithms for kidney replacement therapy (KRT), CV events, other CKD-related complications, and mortality. Risk equations and transition probabilities were primarily sourced from publications produced by large US and international CKD registries. A patient-level, state-transition model was developed with health states defined by the Kidney Disease Improving Global Outcomes categories. Model validation was performed by comparing predicted outcomes with observed outcomes in the source cohorts used in model development (internal validation) and other cohorts (external validation). The CKD-PM demonstrated satisfactory modeling properties. Accurate prediction of all-cause and CV mortality was achieved without calibration, while prediction of CV events through CKD-specific equations required implementation of a calibration factor to balance time-dependent versus baseline risk. Predicted annual changes in estimated glomerular filtration rate (eGFR) and urine albumin-creatinine ratio were acceptable in comparison to external values. A flexible eGFR threshold for KRT equations enabled accurate prediction of these events. This CKD-PM demonstrated reliable modeling properties. Both internal and external validation revealed robust outcomes.
Keywords: Chronic kidney disease; disease progression model; microsimulation; validation.
Conflict of interest statement
L. Gerlier is an employee of IQVIA; IQVIA received consulting fees from Boehringer Ingelheim. A. Uster, L. Muttram, and D. Steubl are employees of Boehringer Ingelheim. A.H. Frankel has received research grants and fees for advisory board attendance from Boehringer Ingelheim, Lilly, AstraZeneca, Menarani, and Bayer. M. Ramos and M. Lamotte were employees of IQVIA at the time of the study. All authors met the criteria for authorship specified by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive honoraria or payments related to the development of the manuscript. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
