Exosomal miR-199a-3p Secreted From Cancer-Associated Adipocytes Promotes Pancreatic Cancer Progression
- PMID: 39431622
- PMCID: PMC11492146
- DOI: 10.1002/cam4.70265
Exosomal miR-199a-3p Secreted From Cancer-Associated Adipocytes Promotes Pancreatic Cancer Progression
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer. Recent studies indicated that cancer-associated adipocytes (CAAs) play crucial roles in tumor progression; however, the precise mechanism remains unknown. Here, we analyzed specific exosomal microRNAs (miRNA) signatures derived from pancreatic CAAs to investigate their role in cancer progression.
Methods: CAAs were generated by co-culturing human adipocytes with human pancreatic cancer cells, and exosomes were isolated from the CAA-conditioned medium (CAA-CM). Small RNA-seq analysis was used to identify differentially expressed miRNAs in these exosomes. The effects of miRNAs on cell proliferation, migration/invasion, and drug sensitivity were examined. Luciferase reporter assays, real-time polymerase chain reaction, and western blotting were performed to investigate the molecular mechanisms of the miRNAs. The clinical relevance of the miRNAs was investigated using publicly available data and our cohort of patients with PDAC.
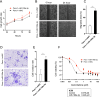
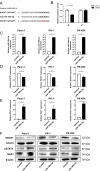
Results: miR-199a-3p expression was significantly increased in CAA-CM-derived exosomes. CAA-derived exosomes transferred miR-199a-3p to pancreatic cancer cells. Transfection with miR-199a-3p increased the proliferation, invasion, migration, and drug resistance of pancreatic cancer cells by downregulating SOCS7, increasing STAT3 phosphorylation, and upregulating SAA1 expression. High tissue miR-199a-3p expression is correlated with poor prognosis in patients with PDAC. Liquid biopsies revealed that exosomal miR-199a-3p could accurately differentiate patients with PDAC from healthy controls. Multivariate survival analysis indicated that miR-199a is an independent prognostic factor for PDAC.
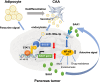
Conclusion: miR-199a-3p in CAA-derived exosomes contributes to the malignant transformation of pancreatic cancer via the SOCS7/STAT3/SAA1 pathway, suggesting its potential as a biomarker and therapeutic target for PDAC.
Keywords: biomarker; cancer‐associated adipocytes; miR‐199a‐3p; pancreatic ductal adenocarcinoma.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Garrido‐Laguna I. and Hidalgo M., “Pancreatic Cancer: From State‐Of‐The‐Art Treatments to Promising Novel Therapies,” Nature Reviews. Clinical Oncology 12 (2015): 319–334, http://www.nature.com/articles/nrclinonc.2015.53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

