Homeostatic Macrophages Prevent Preterm Birth and Improve Neonatal Outcomes by Mitigating In Utero Sterile Inflammation in Mice
- PMID: 39431882
- PMCID: PMC11572957
- DOI: 10.4049/jimmunol.2400467
Homeostatic Macrophages Prevent Preterm Birth and Improve Neonatal Outcomes by Mitigating In Utero Sterile Inflammation in Mice
Abstract
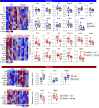
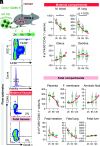
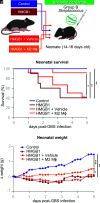
Preterm birth (PTB), often preceded by preterm labor, is a major cause of neonatal morbidity and mortality worldwide. Most PTB cases involve intra-amniotic inflammation without detectable microorganisms, termed in utero sterile inflammation, for which there is no established treatment. In this study, we propose homeostatic macrophages to prevent PTB and adverse neonatal outcomes caused by in utero sterile inflammation. Single-cell atlases of the maternal-fetal interface revealed that homeostatic maternal macrophages are reduced with human labor. M2 macrophage treatment prevented PTB and reduced adverse neonatal outcomes in mice with in utero sterile inflammation. Specifically, M2 macrophages halted premature labor by suppressing inflammatory responses in the amniotic cavity, including inflammasome activation, and mitigated placental and offspring lung inflammation. Moreover, M2 macrophages boosted gut inflammation in neonates and improved their ability to fight systemic bacterial infections. Our findings show that M2 macrophages are a promising strategy to mitigate PTB and improve neonatal outcomes resulting from in utero sterile inflammation.
Copyright © 2024 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures






References
-
- Liu, L., Oza S., Hogan D., Perin J., Rudan I., Lawn J. E., Cousens S., Mathers C., Black R. E.. 2015. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385: 430–440. - PubMed
-
- Bastek, J. A., Gomez L. M., Elovitz M. A.. 2011. The role of inflammation and infection in preterm birth. Clin. Perinatol. 38: 385–406. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

