Skeletal Editing of Mechanically Interlocked Molecules: Nitrogen Atom Deletion from Crown Ether-Dibenzylammonium Rotaxanes
- PMID: 39431981
- PMCID: PMC11528408
- DOI: 10.1021/jacs.4c09066
Skeletal Editing of Mechanically Interlocked Molecules: Nitrogen Atom Deletion from Crown Ether-Dibenzylammonium Rotaxanes
Abstract
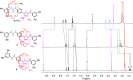
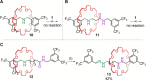
Removing the nitrogen atom from secondary amines while simultaneously linking the remaining fragments is a powerful form of late-stage skeletal editing. Here, we report its use for the deletion of the nitrogen atom of the dibenzylammonium template used to assemble crown ether rotaxanes. The reaction uses an anomeric amide that activates secondary amines to generate a carbon-carbon bond that replaces the amine nitrogen. Despite the potential for dethreading of the intermediate diradical pair, the nitrogen atom was successfully deleted from a series of rotaxane axles as long as the macrocycle could access coconformations that did not inhibit the reaction of the amine group. The skeletally edited interlocked molecules were obtained directly from the parent crown ether-dibenzylammonium rotaxanes in modest yields (23-36%) and characterized by NMR spectroscopy, mass spectrometry, and X-ray crystallography. One skeletally edited rotaxane shows a network of weak CH···O hydrogen bonds between the crown ether and benzylic methylene groups of the axle in the solid state, in place of the crown ether-ammonium binding motif used to assemble the parent, unedited, rotaxane.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Bruns C. J.; Stoddart J. F.. The Nature of the Mechanical Bond: From Molecules to Machines; John Wiley & Sons: Hoboken, NJ, 2017.
-
- Aucagne V.; Hänni K. D.; Leigh D. A.; Lusby P. J.; Walker D. B. Catalytic “click” rotaxanes: A substoichiometric metal-template pathway to mechanically interlocked architectures. J. Am. Chem. Soc. 2006, 128, 2186–2187. 10.1021/ja056903f. - DOI - PubMed
- Crowley J. D.; Goldup S. M.; Lee A.-L.; Leigh D. A.; McBurney R. T. Active metal template synthesis of rotaxanes, catenanes and molecular shuttles. Chem. Soc. Rev. 2009, 38, 1530–1541. 10.1039/b804243h. - DOI - PubMed
- Denis M.; Goldup S. M. The active template approach to interlocked molecules. Nat. Rev. Chem. 2017, 1 (8), 0061.10.1038/s41570-017-0061. - DOI
-
- Segawa Y.; Kuwayama M.; Hijikata Y.; Fushimi M.; Nishihara T.; Pirillo J.; Shirasaki J.; Kubota N.; Itami K. Topological molecular nanocarbons: All-benzene catenane and trefoil knot. Science 2019, 365, 272–276. 10.1126/science.aav5021. - DOI - PubMed
- Segawa Y.; Levine D. R.; Itami K. Topologically unique molecular nanocarbons. Acc. Chem. Res. 2019, 52, 2760–2767. 10.1021/acs.accounts.9b00402. - DOI - PubMed
- Segawa Y.; Kuwayama M.; Itami K. Synthesis and structure of [9]cycloparaphenylene catenane: An all-benzene catenane consisting of small rings. Org. Lett. 2020, 22, 1067–1070. 10.1021/acs.orglett.9b04599. - DOI - PubMed
-
- Hannam J. S.; Lacy S. M.; Leigh D. A.; Saiz C. G.; Slawin A. M. Z.; Stitchell S. G. Controlled submolecular translational motion in synthesis: A mechanically interlocking auxiliary. Angew. Chem., Int. Ed. 2004, 43, 3260–3264. 10.1002/anie.200353606. - DOI - PubMed
- Chao S.; Romuald C.; Fournel-Marotte K.; Clavel C.; Coutrot F. A strategy utilizing a recyclable macrocycle transporter for the efficient synthesis of a triazolium-based [2]rotaxane. Angew. Chem., Int. Ed. 2014, 53, 6914–6919. 10.1002/anie.201403765. - DOI - PubMed
- Waelès P.; Clavel C.; Fournel-Marotte K.; Coutrot F. Synthesis of triazolium-based mono- and tris-branched [1]rotaxanes using a molecular transporter of dibenzo-24-crown-8. Chem. Sci. 2015, 6, 4828–4836. 10.1039/C5SC01722J. - DOI - PMC - PubMed
- Riss-Yaw B.; Clavel C.; Laurent P.; Waelès P.; Coutrot F. The importance of length and flexibility of macrocycle-containing molecular translocators for the synthesis of improbable [2]rotaxanes. Chem. -Eur. J. 2018, 24, 13659–13666. 10.1002/chem.201802831. - DOI - PubMed
- Waelès P.; Gauthier M.; Coutrot F. Challenges and Opportunities in the Post-Synthetic Modification of Interlocked Molecules. Angew. Chem., Int. Ed. 2021, 60, 16778–16799. 10.1002/anie.202007496. - DOI - PubMed
- Koehler V.; Gauthier M.; Yao C.; Fournel-Marotte K.; Waelès P.; Kauffmann B.; Huc I.; Coutrot F.; Ferrand Y. [3]Foldarotaxane-mediated synthesis of an improbable [2]rotaxane. Chem. Commun. 2022, 58, 8618–8621. 10.1039/D2CC03066G. - DOI - PubMed
- de Juan A.; Lozano D.; Heard A. W.; Jinks M. A.; Suarez J. M.; Tizzard G. J.; Goldup S. M. A chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes. Nat. Chem. 2022, 14, 179–187. 10.1038/s41557-021-00825-9. - DOI - PMC - PubMed
- Tsai C.-Y.; Cheng H.-T.; Chiu S.-H. Improbable rotaxanes constructed from surrogate malonate rotaxanes as encircled methylene synthons. Angew. Chem. Int. Ed. 2023, 62, e20230897410.1002/anie.202308974. - DOI - PubMed
- Bu A.; Gao J.-N.; Chen Y.; Xiao H.; Li H.; Tung C.-H.; Wu L.-Z.; Cong H. Modular synthesis of improbable rotaxanes with all-benzene scaffolds. Angew. Chem. Int. Ed. 2024, 63, e20240183810.1002/anie.202401838. - DOI - PubMed
- Saura-Sanmartin A. Synthesis of ‘impossible’ rotaxanes. Chem. -Eur. J. 2024, 30, e20230402510.1002/chem.202304025. - DOI - PubMed
-
- Kolchinski A. G.; Busch D. H.; Alcock N. W. Gaining control over molecular threading: Benefits of second coordination sites and aqueous–organic interfaces in rotaxane synthesis. J. Chem. Soc., Chem. Commun. 1995, 1289–1291. 10.1039/C39950001289. - DOI
- Ashton P. R.; Glink P. T.; Stoddart J. F.; Tasker P. A.; White A. J. P.; Williams D. J. Chem. -Eur. J. 1996, 2, 729–735.
- Thibeault D.; Morin J.-F. Recent advances in the synthesis of ammonium-based rotaxanes. Molecules 2010, 15, 3709–3730. 10.3390/molecules15053709. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

