NFAT5 governs cellular plasticity-driven resistance to KRAS-targeted therapy in pancreatic cancer
- PMID: 39432061
- PMCID: PMC11497412
- DOI: 10.1084/jem.20240766
NFAT5 governs cellular plasticity-driven resistance to KRAS-targeted therapy in pancreatic cancer
Abstract
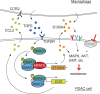
Resistance to KRAS therapy in pancreatic ductal adenocarcinoma (PDAC) involves cellular plasticity, particularly the epithelial-to-mesenchymal transition (EMT), which poses challenges for effective targeting. Chronic pancreatitis, a known risk factor for PDAC, elevates TGFβ levels in the tumor microenvironment (TME), promoting resistance to KRAS therapy. Mechanistically, TGFβ induces the formation of a novel protein complex composed of SMAD3, SMAD4, and the nuclear factor NFAT5, triggering EMT and resistance by activating key mediators such as S100A4. Inhibiting NFAT5 attenuates pancreatitis-induced resistance to KRAS inhibition and extends mouse survival. Additionally, TGFβ stimulates PDAC cells to secrete CCL2, recruiting macrophages that contribute to KRAS bypass through paracrine S100A4. Our findings elucidate the role of TGFβ signaling in EMT-associated KRAS therapy resistance and identify NFAT5 as a druggable target. Targeting NFAT5 could disrupt this regulatory network, offering a potential avenue for preventing resistance in PDAC.
© 2024 Deng et al.
Conflict of interest statement
Disclosures: P. Hou reported a patent to U.S. Provisional Patent Application No. 63/641,226 pending. No other disclosures were reported.
Figures














References
-
- Adachi, Y., Ito K., Hayashi Y., Kimura R., Tan T.Z., Yamaguchi R., and Ebi H.. 2020. Epithelial-to-mesenchymal transition is a cause of both intrinsic and acquired resistance to KRAS G12C inhibitor in KRAS G12C-mutant non-small cell lung cancer. Clin. Cancer Res. 26:5962–5973. 10.1158/1078-0432.CCR-20-2077 - DOI - PubMed
-
- Bardeesy, N., Cheng K.H., Berger J.H., Chu G.C., Pahler J., Olson P., Hezel A.F., Horner J., Lauwers G.Y., Hanahan D., and DePinho R.A.. 2006. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 20:3130–3146. 10.1101/gad.1478706 - DOI - PMC - PubMed
-
- Byers, L.A., Diao L., Wang J., Saintigny P., Girard L., Peyton M., Shen L., Fan Y., Giri U., Tumula P.K., et al. . 2013. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 19:279–290. 10.1158/1078-0432.CCR-12-1558 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

