Cangrelor versus crushed ticagrelor in patients with acute myocardial infarction and cardiogenic shock: rationale and design of the randomised, double-blind DAPT-SHOCK-AMI trial
- PMID: 39432252
- PMCID: PMC11472137
- DOI: 10.4244/EIJ-D-24-00203
Cangrelor versus crushed ticagrelor in patients with acute myocardial infarction and cardiogenic shock: rationale and design of the randomised, double-blind DAPT-SHOCK-AMI trial
Abstract
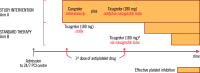
Cardiogenic shock (CS) is a devastating and fatal complication of acute myocardial infarction (AMI). CS can affect the pharmacokinetics and pharmacodynamics of medications. The unique properties of cangrelor make it the optimal P2Y12 inhibitor for CS-AMI, in terms of both efficacy and safety. The DAPT-SHOCK-AMI trial (ClinicalTrials.gov: NCT03551964; EudraCT: 2018-002161-19) will assess the benefits of cangrelor in patients with an initial CS-AMI undergoing primary angioplasty. This randomised, multicentre, placebo-controlled trial of approximately 550 patients (with an allowed 10% increase) in 5 countries using a double-blind design will compare initial P2Y12 inhibitor treatment strategies in patients with CS-AMI of (A) intravenous cangrelor and (B) ticagrelor administered as crushed tablets at a loading dose of 180 mg. The primary clinical endpoint is a composite of all-cause death, myocardial infarction (MI), or stroke within 30 days. The main secondary endpoints are (1) the net clinical endpoint, defined as death, MI, urgent revascularisation of the infarct-related artery, stroke, or major bleeding as defined by the Bleeding Academic Research Consortium criteria; (2) cardiovascular-related death, MI, urgent revascularisation, or heart failure; (3) heart failure; and (4) cardiovascular-related death, all (1-4) within 1 year after study enrolment. A platelet reactivity study that tests the laboratory antiplatelet benefits of cangrelor, when given in addition to standard antiplatelet therapy, will be conducted using vasodilator-stimulated phosphoprotein phosphorylation. The primary laboratory endpoints are the periprocedural rate of onset and the proportion of patients who achieve effective P2Y12 inhibition. The DAPT-SHOCK-AMI study is the first randomised trial to evaluate the benefits of cangrelor in patients with CS-AMI.
Conflict of interest statement
Z. Motovska discloses the following relationships: research funding related to the implementation of the DAPT-SHOCK-AMI study: the Ministry of Health of the Czech Republic Grant No. NV19-02-00086; the Charles University Czech Republic: research programmes PROGRESS Q 38 and COOPERATIO - Cardiovascular Science, research support from Donatio Universitatis Carolinae, the Charles University 4EU+ mini-grant (No. 4EU+/23/F1/04); Programme EXCELES, ID Project No. LX22NPO5104 - funded by the European Union - Next Generation EU; advisory boards: AstraZeneca, Bayer, Boehringer Ingelheim, Chiesi, Sanofi; research funds for the institution or honoraria from: AstraZeneca, Amgen, Bayer, Czech Society of Cardiology, Idorsia, Janssen, RITA-MI 2 Grant agreement ID: 899991, HORIZON 2020-EU 3.1.1; meeting attendance support: Czech Society of Cardiology, Boehringer Ingelheim, Pfizer. D.L. Bhatt discloses the following relationships - advisory board: Angiowave, Bayer, Boehringer Ingelheim, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Stasys; board of directors: American Heart Association New York City, Angiowave (stock options), Bristol-Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock); consultant: Broadview Ventures, GlaxoSmithKline, Hims, SFJ, Youngene; data monitoring committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (chair, PEITHO trial), Cleveland Clinic, Contego Medical (chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical; for ALLAY-HF, funded by Alleviant Medical), Novartis, Population Health Research Institute, Rutgers University (for the NIH-funded MINT trial); honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, comprehensive review of interventional cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), WebMD (CME steering committees), Wiley (steering committee); other: Clinical Cardiology (Deputy Editor); patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women’s Hospital who assigned to Lexicon; neither he nor Brigham and Women’s Hospital receive any income from this patent); research funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; royalties: Elsevier (Editor, Braunwald’s Heart Disease); site co-investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; trustee: American College of Cardiology; unfunded research: FlowCo. P. Kala discloses the following relationships - advisory board: Boston Scientific, Edwards Lifesciences, Chiesi, Novartis, Sanofi, Servier; speaker and consultancy fees: Abbott, Boston Scientific, Servier, Zentiva. T. Giesler discloses the following relationships: speaker and consultancy fees from AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, Ferrer/Chiesi, Novartis, Medtronic, and Edwards Lifesciences; research grants from Bayer, Bristol-Myers Squibb/Pfizer, Ferrer/Chiesi, Medtronic, and Edwards Lifesciences. J. Belohlavek discloses consulting fees from Abiomed and Resuscitec; honoraria from Novartis, Boehringer Ingelheim, and AstraZeneca. G. Montalescot declares research funds for the institution or honoraria from: Abbott, Amgen, AstraZeneca, Ascendia, Bayer, BMS, Boehringer Ingelheim, Boston Scientific, Celecor, CSL Behring, Idorsia, Lilly, Medpace, Novartis, Novo Nordisk, Opalia, Pfizer, Sanofi, Terumo. J. Matejka declares speaker honoraria from AstraZeneca and Servier. C.B. Olivier reports research support from Deutsche Forschungsgemeinschaft, Deutsche Herzstiftung, Freiburg University, Else Kröner-Fresenius Stiftung, and Haemonetics; honoraria: Bayer Vital GmbH, BMS, Boehringer Ingelheim, Daiichi Sankyo, Ferrer, Idorsia, and Janssen. P. Ostadal discloses the following relationships - advisory board: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Sanofi; speaker and consultancy fees: Abiomed, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Edwards Lifesciences, Fresenius, Getinge, Novartis, Pfizer, Sanofi, Servier. G. Ducrocq discloses speaker and/or consulting fees from Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boston Scientific, BMS, Novo Nordisk, Sanofi, CEC, DSMB; steering committee in Amgen, Novo Nordisk, Janssen; proctoring for Boston Scientific; travel fees from Sanofi; ownership interest in Bioquantis. M. Karpisek discloses: co-investigator of BioVendor, BioLab Assays. P. Tomasov discloses travel fees from Boston Scientific and Euromedical. J. Kubica discloses lecture honoraria from AstraZeneca and Ferrer. The other authors have no conflicts of interest to declare.
Figures



References
-
- Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi JY, Christensen H, Cirillo M, Cooper L, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadele Atnafu, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. - PMC - PubMed
-
- Schrage B, Becher PM, Goßling A, Savarese G, Dabboura S, Yan I, Beer B, Söffker G, Seiffert M, Kluge S, Kirchhof P, Blankenberg S, Westermann D. Temporal trends in incidence, causes, use of mechanical circulatory support and mortality in cardiogenic shock. ESC Heart Fail. 2021;8:1295–303. - PMC - PubMed
-
- Hunziker L, Radovanovic D, Jeger R, Pedrazzini G, Cuculi F, Urban P, Erne P, Rickli H, Pilgrim T AMIS Plus Registry Investigators are listed in alphabetic order with the names of the local principal investigators. Twenty-Year Trends in the Incidence and Outcome of Cardiogenic Shock in AMIS Plus Registry. Circ Cardiovasc Interv. 2019;12:e007293. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

