Outcomes with revascularisation versus conservative management of participants with 3-vessel coronary artery disease in the ISCHEMIA trial
- PMID: 39432255
- PMCID: PMC11472139
- DOI: 10.4244/EIJ-D-24-00240
Outcomes with revascularisation versus conservative management of participants with 3-vessel coronary artery disease in the ISCHEMIA trial
Abstract
Background: Whether revascularisation (REV) improves outcomes in patients with three-vessel coronary artery disease (3V-CAD) is uncertain.
Aims: Our objective was to evaluate outcomes with REV (percutaneous coronary intervention [PCI] or coronary artery bypass graft surgery [CABG]) versus medical therapy in patients with 3V-CAD.
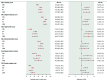
Methods: ISCHEMIA participants with 3V-CAD on coronary computed tomography angiography without prior CABG were included. Outcomes following initial invasive management (INV) with REV (PCI or CABG) versus initial conservative management (CON) with medical therapy alone were evaluated. Regression modelling was used to estimate the outcomes if all participants were to undergo prompt REV versus those assigned to CON. Outcomes were cardiovascular (CV) death/myocardial infarction (MI), death, CV death, and quality of life. Bayesian posterior probability for benefit (Pr [benefit]) for 1 percentage point lower 4-year rates with REV versus CON were evaluated.
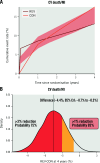
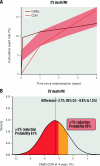
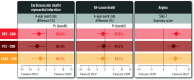
Results: Among 1,236 participants with 3V-CAD (612 INV/624 CON), REV was associated with lower 4-year CV death/MI (adjusted 4-year difference: -4.4, 95% credible interval [CrI] -8.7 to -0.3 percentage points, Pr [benefit]=94.8%) when compared with CON, with similar results for PCI versus CON (-5.8, 95% CrI: -10.8 to -0.5 percentage points, Pr [benefit]=96.4%) and CABG versus CON (-3.7, 95% CrI: -8.8 to 1.5 percentage points, Pr [benefit]=84.7%). Adjusted 4-year REV versus CON differences were as follows: death -1.2 (95% CrI: -4.7 to 2.2) percentage points, CV death -2.3 (95% CrI: -5.5 to 0.8) percentage points, with similar results for PCI and for CABG. The Pr (benefit) for death with REV (PCI or CABG) versus CON was 49-63%. The adjusted 12-month Seattle Angina Questionnaire-7 summary score differences favoured REV: REV versus CON 4.6 (95% CrI: 2.7-6.4) percentage points; PCI versus CON 3.6 (95% CrI: 1.2-5.8) percentage points and CABG versus CON 4.3 (95% CrI: 1.5-6.9) percentage points with high Pr (benefit).
Conclusions: In participants with 3V-CAD, REV (either PCI or CABG) was associated with a lower 4-year CV death/MI rate and improved quality of life, with similar results for PCI versus CON and CABG versus CON. The differences in all-cause mortality between REV and CON were small with wide confidence intervals. (ClinicalTrials.gov: NCT01471522).
Conflict of interest statement
S. Bangalore reports grants from the National Heart, Lung, and Blood Institute during the conduct of the study; grants and personal fees from Abbott; personal fees from Biotronik, Pfizer, Amgen, and Reata outside of the submitted work. G. Rhodes reports NIH funding. D.J. Maron reports grants from the National Heart, Lung, and Blood Institute during the conduct of the study. R. Anthopolos reports grants from the National Heart, Lung, and Blood Institute during the conduct of the study. S.M. O’Brien reports grants from the National Heart, Lung, and Blood Institute during the conduct of the study. D.B. Mark reports grants from National Heart, Lung, and Blood Institute during the conduct of the study; and grants from HeartFlow and Merck, outside the submitted work. H.R. Reynolds reports grants from the National Heart, Lung, and Blood Institute during the conduct of the study; and non-financial support from Abbott, Siemens, and BioTelemetry, outside of the submitted work. J.A. Spertus reports grants from National Heart, Lung, and Blood Institute during the conduct of the study; personal fees from Bayer, Novartis, AstraZeneca, Amgen, Janssen, and United Healthcare; and grants from American College of Cardiology, outside the submitted work; in addition, he has a patent copyright to the Seattle Angina Questionnaire with royalties paid; is on the Board of Directors for Blue Cross Blue Shield of Kansas City; and reports equity in Health Outcomes Sciences. G.W. Stone has received speaker honoraria from Medtronic, Pulnovo, and Infraredx; has served as a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Abiomed, Ancora, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Cardiomech, Gore Medical, Amgen, Adona Medical, and Millennia Biopharma; and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWAVE, Orchestra Biomed, Aria, Cardiac Success, Valfix, and Xenter; his daughter is an employee at Medtronic; for institutional disclosure, his employer, Mount Sinai Hospital, receives research support from Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Philips, Biosense Webster, Shockwave Medical, Vascular Dynamics, Pulnovo, and V-Wave. H.D. White reports grants from National Heart, Lung, and Blood Institute during the conduct of the study; reports receiving grant support paid to the institution and fees for serving on a steering committee for the ODYSSEY OUTCOMES trial (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) from Sanofi-Aventis and Regeneron Pharmaceuticals; for the ACCELERATE study (A Study of Evacetrapib in High-Risk Vascular Disease) from Eli Lilly; for the STRENGTH trial (Outcomes Study to Assess Statin Residual Risk Reduction With EpaNova in High CV Risk Patients With Hypertriglyceridemia) from Omthera Pharmaceuticals; for the HEART-FID study (Randomized Placebo-Controlled Trial of FCM as Treatment for Heart Failure With Iron Deficiency) from American Regent; for the CAMELLIA-TIMI study (A Study to Evaluate the Effect of Long-term Treatment With BELVIQ [Lorcaserin HC] on the Incidence of Major Adverse Cardiovascular Events and Conversion to Type 2 Diabetes Mellitus in Obese and Overweight Subjects With Cardiovascular Disease or Multiple Cardiovascular Risk Factors) from Eisai Inc; for the dal-GenE study (Effect of Dalcetrapib vs Placebo on CV Risk in a Genetically Defined Population With a Recent ACS) from DalCor Pharma UK Inc; for the AEGIS-II study from CSL Behring; for the SCORED trial (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) and the SOLOIST-WHF trial (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type2 Diabetes Post Worsening Heart Failure) from Sanofi-Aventis Australia Pty Ltd; and for the CLEAR Outcomes Study (Evaluation of Major Cardiovascular Events in Patients With, or at High Risk for, Cardiovascular Disease Who Are Statin Intolerant Treated With Bempedoic Acid. [ETC-1002] or Placebo) from Esperion Therapeutics Inc; he was on the advisory board for Genentech, Inc.; and received lecture fees from AstraZeneca. Y. Xu reports grants from the National Heart, Lung, and Blood Institute during the conduct of the study. J.S. Hochman is the PI for the ISCHEMIA trial for which, in addition to support by the National Heart, Lung, and Blood Institute grant, devices and medications were provided by Abbott, Medtronic, Abbott Laboratories (formerly St. Jude Medical, Inc.), Royal Philips NV (formerly Volcano Corporation), Arbor Pharmaceuticals, LLC, AstraZeneca Pharmaceuticals, LP, Merck Sharp & Dohme Corp., Omron Healthcare, Inc., Sunovion Pharmaceuticals, Inc., Espero BioPharma, and Amgen, Inc.; and received financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. The other authors have no conflicts of interest to declare.
Figures

References
-
- Rajkumar CA, Foley MJ, Ahmed-Jushuf F, Nowbar AN, Simader FA, Davies JR, O’Kane PD, Haworth P, Routledge H, Kotecha T, Gamma R, Clesham G, Williams R, Din J, Nijjer SS, Curzen N, Ruparelia N, Sinha M, Dungu JN, Ganesananthan S, Khamis R, Mughal L, Kinnaird T, Petraco R, Spratt JC, Sen S, Sehmi J, Collier DJ, Sohaib A, Keeble TR, Cole GD, Howard JP, Francis DP, Shun-Shin MJ, Al-Lamee RK ORBITA-2 Investigators. A Placebo-Controlled Trial of Percutaneous Coronary Intervention for Stable Angina. N Engl J Med. 2023;389:2319–30. - PMC - PubMed
-
- Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O’Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE COURAGE Trial Research Group; Mancini GB. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–87. - PubMed
-
- Spertus JA, Jones PG, Maron DJ, O’Brien SM, Reynolds HR, Rosenberg Y, Stone GW, Harrell FE, Boden WE, Weintraub WS, Baloch K, Mavromatis K, Diaz A, Gosselin G, Newman JD, Mavromichalis S, Alexander KP, Cohen DJ, Bangalore S, Hochman JS, Mark DB ISCHEMIA Research Group. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. N Engl J Med. 2020;382:1408–19. - PMC - PubMed
-
- Writing Committee Members Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, Fremes SE, Gaudino MF, Goldberger ZD, Grant MC, Jaswal JB, Kurlansky PA, Mehran R, Metkus TS Jr, Nnacheta LC, Rao SV, Sellke FW, Sharma G, Yong CM, Zwischenberger BA. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:197–215.
-
- Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, Dixon DL, Fearon WF, Hess B, Johnson HM, Kazi DS, Kolte D, Kumbhani DJ, LoFaso J, Mahtta D, Mark DB, Minissian M, Navar AM, Patel AR, Piano MR, Rodriguez F, Talbot AW, Taqueti VR, Thomas RJ, van Diepen, Wiggins B, Williams MS Peer Review Committee Members. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023;148:e9–119. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

