Development and Validation of a Clinical Score to Predict Epilepsy After Cerebral Venous Thrombosis
- PMID: 39432281
- PMCID: PMC11581563
- DOI: 10.1001/jamaneurol.2024.3481
Development and Validation of a Clinical Score to Predict Epilepsy After Cerebral Venous Thrombosis
Abstract
Importance: One of 10 patients develop epilepsy in the late phase after cerebral venous thrombosis (CVT) diagnosis but predicting the individual risk is difficult.
Objective: To develop and externally validate a prognostic score to estimate the individual risk of post-CVT epilepsy.
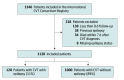
Design, setting, and participants: This observational cohort study included both retrospective and prospective patients enrolled from 1994 through 2022. For development of the DIAS3 score, data from the International CVT Consortium (n = 1128), a large international hospital-based multicenter CVT cohort, were used. For validation, data from 2 independent multicenter cohorts, the ACTION-CVT (n = 543) and the Israel CVT study (n = 556), were used. Of 2937 eligible, consecutively enrolled adult patients with radiologically verified CVT, 710 patients with a history of epilepsy prior to CVT, follow-up less than 8 days, and missing late seizure status were excluded.
Exposure: The prediction score (DIAS3) was developed based on available literature and clinical plausibility and consisted of 6 readily available clinical variables collected during the acute phase: decompressive hemicraniectomy, intracerebral hemorrhage at presentation, age, seizure(s) in the acute phase (excluding status epilepticus), status epilepticus in the acute phase, and subdural hematoma at presentation.
Main outcome and measure: Time to a first late seizure, defined as occurring more than 7 days after diagnosis of CVT.
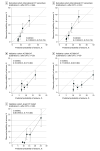
Results: Of 1128 patients included in the derivation cohort (median age, 41 [IQR, 30-53] years; 805 women [71%]), 128 (11%) developed post-CVT epilepsy during a median follow-up of 12 (IQR, 3-26) months. According to the DIAS3 score, the predicted 1-year and 3-year risk of epilepsy in individual patients ranged from 7% to 68% and 10% to 83%, respectively. Internal and external validation showed adequate discrimination in the derivation cohort (1 year and 3 years: C statistic, 0.74; 95% CI, 0.70-0.79) and the 2 independent validation cohorts, (ACTION-CVT) 1 year: C statistic, 0.76; 95% CI, 0.67-0.84; 3 years: C statistic, 0.77; 95% CI, 0.66-0.84; and Israel CVT study 1 year: C statistic, 0.80; 95% CI, 0.75-0.86. Calibration plots indicated adequate agreement between predicted and observed risks.
Conclusions and relevance: The DIAS3 score (freely available online) is a simple tool that can help predict the risk of post-CVT epilepsy in individual patients. The model can improve opportunities for personalized medicine and may aid in decision-making regarding antiseizure medication, patient counseling, and facilitation of research on epileptogenesis in CVT.
Conflict of interest statement
Figures
References
-
- Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators . Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664-670. doi: 10.1161/01.STR.0000117571.76197.26 - DOI - PubMed

