The Drosophila circadian clock gene cycle controls the development of clock neurons
- PMID: 39432537
- PMCID: PMC11527286
- DOI: 10.1371/journal.pgen.1011441
The Drosophila circadian clock gene cycle controls the development of clock neurons
Abstract
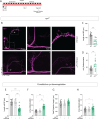
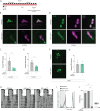
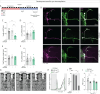
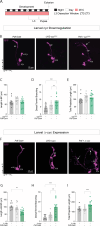
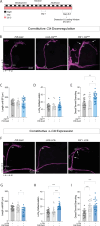
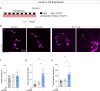
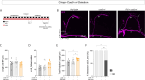
Daily behavioral and physiological rhythms are controlled by the brain's circadian timekeeping system, a synchronized network of neurons that maintains endogenous molecular oscillations. These oscillations are based on transcriptional feedback loops of clock genes, which in Drosophila include the transcriptional activators Clock (Clk) and cycle (cyc). While the mechanisms underlying this molecular clock are very well characterized, the roles that the core clock genes play in neuronal physiology and development are much less understood. The Drosophila timekeeping center is composed of ~150 clock neurons, among which the four small ventral lateral neurons (sLNvs) are the most dominant pacemakers under constant conditions. Here, we show that downregulating the clock gene cyc specifically in the Pdf-expressing neurons leads to decreased fasciculation both in larval and adult brains. This effect is due to a developmental role of cyc, as both knocking down cyc or expressing a dominant negative form of cyc exclusively during development lead to defasciculation phenotypes in adult clock neurons. Clk downregulation also leads to developmental effects on sLNv morphology. Our results reveal a non-circadian role for cyc, shedding light on the additional functions of circadian clock genes in the development of the nervous system.
Copyright: © 2024 Biondi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

