QSOX1 facilitates dormant esophageal cancer stem cells to evade immune elimination via PD-L1 upregulation and CD8 T cell exclusion
- PMID: 39432781
- PMCID: PMC11536095
- DOI: 10.1073/pnas.2407506121
QSOX1 facilitates dormant esophageal cancer stem cells to evade immune elimination via PD-L1 upregulation and CD8 T cell exclusion
Abstract
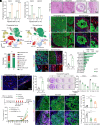
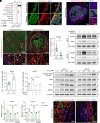
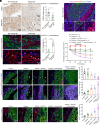
Dormant cancer stem cells (DCSCs) exhibit characteristics of chemotherapy resistance and immune escape, and they are a crucial source of tumor recurrence and metastasis. However, the underlying mechanisms remain unrevealed. We demonstrate that enriched Gzmk+ CD8+ T cells within the niche of esophageal DCSCs restrict the outgrowth of tumor mass. Nonetheless, DCSCs can escape immune elimination by enhancing PD-L1 signaling, thereby maintaining immune equilibrium. Quiescent fibroblast-derived quiescin sulfhydryl oxidase 1 (QSOX1) promotes the expression of PD-L1 and its own expression in DCSCs by elevating the level of reactive oxygen species. Additionally, high QSOX1 in the dormant tumor niche contributes to the exclusion of CD8+ T cells. Conversely, blocking QSOX1 with Ebselen in combination with anti-PD-1 and chemotherapy can effectively eradicate residual DCSCs by reducing PD-L1 expression and promoting CD8+ T cell infiltration. Clinically, high expression of QSOX1 predicts a poor response to anti-PD-1 treatment in patients with esophageal cancer. Thus, our findings reveal a mechanism whereby QSOX1 promotes PD-L1 upregulation and T cell exclusion, facilitating the immune escape of DCSCs, and QSOX1 inhibition, combined with immunotherapy and chemotherapy, represents a promising therapeutic approach for eliminating DCSCs and preventing recurrence.
Keywords: PD-1/PD-L1; T cell exclusion; esophageal cancer stem cell; reactive oxygen species; tumor dormancy.
Conflict of interest statement
Competing interests statement:L.L. and J.-R.W. are inventors on a pending patent related to this work (patentee: Sun Yat-sen Memorial Hospital, 11 June 2024; Application No.: 202410743238.7). The other authors declare that they have no other competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

