Unveiling the secrets of lotus seed longevity: insights into adaptive strategies for extended storage
- PMID: 39432815
- PMCID: PMC12351163
- DOI: 10.1093/jxb/erae432
Unveiling the secrets of lotus seed longevity: insights into adaptive strategies for extended storage
Abstract
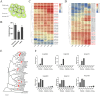
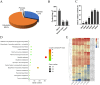
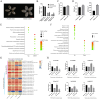
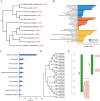
Seed longevity is crucial for long-term storage, but prolonged unfavorable conditions can lead to loss of viability. This study integrated theoretical and experimental techniques to elucidate the inherent mechanisms underlying the unique ability of lotus seeds to maintain stable viability over many years. Transcriptome analysis and microscopy revealed a sturdy structure of the lotus seed pericarp, which predominantly expressed cellulose synthase genes involved in cell wall biogenesis. The cotyledon serves as a nutrient source for seeds during long-term storage. Additionally, the inactivation of chlorophyll degradation pathways may allow for the retention of chlorophyll in the lotus seed plumule, potentially enhancing the environmental adaptability of lotus seedlings. Reduced abundance of transcripts corresponding to heat shock protein genes could impact protein processing and consequently diminish the vitality of aging lotus seeds. Moreover, an expansion in the number of seed maturation and defense response genes was observed in the lotus genome compared with 11 other species, which might represent an adaptive strategy against long-term adverse storage conditions. Overall, these findings are crucial for understanding the mechanisms underlying lotus seed longevity and may inform future improvements in the extended storage periods of seed crops.
Keywords: Ancient lotus seed; accelerated aging; genome; longevity; lotus; transcriptome.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Experimental Biology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
The authors declare that they have no conflicts of interest in relation to this work.
Figures



Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
A Group 6 LEA Protein Plays Key Roles in Tolerance to Water Deficit, and in Maintaining the Glassy State and Longevity of Seeds.Plant Cell Environ. 2025 Sep;48(9):6874-6896. doi: 10.1111/pce.15649. Epub 2025 Jun 5. Plant Cell Environ. 2025. PMID: 40474454 Free PMC article.
-
Comparative transcriptome analysis reveals the potential mechanism of seed germination promoted by trametenolic acid in Gastrodia elata Blume.Sci Rep. 2025 Jul 24;15(1):26869. doi: 10.1038/s41598-025-12269-z. Sci Rep. 2025. PMID: 40702084 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Global consensus on optimal exercise recommendations for enhancing healthy longevity in older adults (ICFSR).J Nutr Health Aging. 2025 Jan;29(1):100401. doi: 10.1016/j.jnha.2024.100401. Epub 2025 Jan 1. J Nutr Health Aging. 2025. PMID: 39743381 Free PMC article. Review.
References
-
- Ballesteros D, Pritchard HW, Walters C.. 2020. Dry architecture: towards the understanding of the variation of longevity in desiccation-tolerant germplasm. Seed Science Research 30, 142–155.
-
- Bernard A, Domergue F, Pascal S, Jetter R, Renne C, Faure JD, Haslam RP, Napier JA, Lessire R, Joubès J.. 2012. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. The Plant Cell 24, 3106–3118. - PMC - PubMed
-
- Börner A, Nagel M, Agacka-Mołdoch M, Gierke PU, Oberforster M, Albrecht T, Mohler V.. 2018. QTL analysis of falling number and seed longevity in wheat (Triticum aestivum L.). Journal of Applied Genetics 59, 35–42. - PubMed
-
- Buitink J, Leprince O.. 2008. Intracellular glasses and seed survival in the dry state. Comptes Rendus Biologies 331, 788–795. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

